Synthesis of activated carbons from water hyacinth biomass and its application as adsorbents in water pollution control Scientific paper
Main Article Content
Abstract
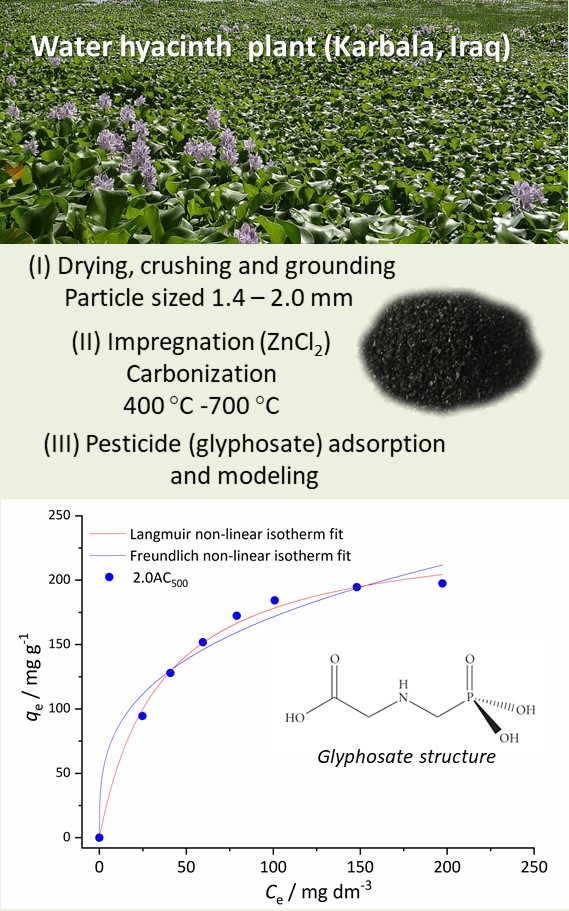
The water hyacinth biomass was used for the synthesis of activated carbons in a process of chemical activation with ZnCl2, followed by controlled pyrolysis. The applied impregnation weight ratios ZnCl2 and dry hyacinth biomass were in the range of 0.5–3.5. The carbonization was conducted at four different temperatures (400–700 °C) under an inert atmosphere. The highest yield of activated carbon was obtained for the impregnation ratio of 0.5 and carbonization temperature of 400 °C. The samples were characterized using elemental analysis, adsorption–desorption isotherms of nitrogen and SEM analysis. The activated carbon obtained with an impregnation ratio 2.0 and carbonization temperature of 500 °C (2.0AC500) showed the highest values of specific surface area and total pore volume of 1317 m2 g-1 and 0.697 cm3 g-1, respectively. The adsorption of glyphosate, pesticide with a strong negative environmental impact, was a fast process, with the equilibrium time of 120 min. The adsorption isotherms were fitted with Langmuir and Freundlich model. The Langmuir adsorption capacity of qmax = 240.8 mg g-1 for 2.0AC500 classified the selected adsorbent as a very efficient one. The tested adsorption process followed the kinetics of the pseudo-second-order model.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
M. A. Bote, V. R. Naik, K. B. Jagadeeshgouda, Mater. Sci. Energy Technol. 3 (2020) 397 (https://doi.org/10.1016/j.mset.2020.02.003)
M. Bilal, J. Ali, N. Hussain, M. Umar, S. Shujah, D. Ahmad, J. Serb. Chem. Soc. 85 (2020) 265 (https://doi.org/10.2298/JSC181108001B)
A. Saning, S. Herou, D. Dechtrirat, C. Ieosakulrat, P. Pakawatpanurut, S. Kaowphong, C. Thanachayanont, M. M. Titirici, L. Chuenchom, RSC Adv. 9 (2019) 24248 (https://doi.org/10.1039/C9RA03873F)
A. Boonpoke, J. Environ. Biol. 36 (2015) 1143 (http://www.jeb.co.in/journal_issues/201509_sep15/paper_15.pdf)
C. A. Riyanto, E. Prabalaras, J. Phys.: Conf. Ser. 1307 (2019) 012002 (https://doi.org/10.1088/1742-6596/1307/1/012002)
M. I. Din, S. Ashraf, A. Intisar, Sci. Prog. 100 (2017) 299 (https://doi.org/10.3184/003685017X14967570531606)
A. Regti, M. R. Laamari, S. E. Stiriba, M. El-Haddad, J. Assoc. Arab Univ. Basic Appl. Sci. 24 (2017) 10 (https://doi.org/10.1016/j.jaubas.2017.01.003)
Z. Hu, M. P. Srinivasan, Micropor. Mesopor. Mater. 43 (2001) 267 (https://doi.org/ 10.1016/S1387-1811(00)00355-3)
Z. Yue, J. Economy, Micropor.Mesopor. Mater. 96 (2006) 314 (https://doi.org/10.1016/j.micromeso.2006.07.025)
K. Wu, B. Gao, J. Su, X. Peng, X. Zhang, J. Fu, P. K. Chu, RSC Adv. 6 (2016) 29996 (https://doi.org/10.1039/C5RA25098F )
Y. Huang, L. Shunxing, C. Jianhua, Z. Xueliang, C. Yiping, Appl. Surf. Sci. 293 (2014) 160 (https://doi.org/10.1016/j.apsusc.2013.12.123)
S. T. Senthilkumar, R. Kalai Selvan, Y. S. Lee, J. S. Melo, J. Mater. Chem., A 1 (2013) 1086 (https://doi.org/10.1039/c2ta00210h1086)
M. T. Scholtz, E. Voldner, A. C. McMillan, B. J. Van Heyst, Atmos. Environ. 36 (2002) 5005 (https://doi.org/10.1016/S1352-2310(02)00570-8)
M. Schweizer, K. Brilisauer, R. Triebskorn, K. Forchhammer, H. R. Köhler, Peer J. 7 (2019) 7094 (https://doi.org/10.7717/peerj.7094)
W. Morley, S. Seneff, Surg. Neurol. Int. 5 (2014) 134731 (https://doi.org/10.4103/2152-7806.134731)
T. H. Liou, Chem. Eng. J. 158 (2010) 129 (https://doi.org/10.1016/j.cej.2009.12.016)
J. Rouquerol, P. Llewellyn, F. Rouquerol, Stud. Surf. Sci. Catal. 160 (2007) 49 (https://doi.org/10.1016/S0167-2991(07)80008-5)
S. J. Gregg, K. S. W. Sing, Adsorption, Surface Area, and Porosity 2, Academic Press, London, 1982, pp. 41–105 (https://doi.org/10.1002/bbpc.19820861019)
F. Rouquerol, J. Rouquerol, K. Sing, Absorption by powders and porous solids, Principles, Methodology and Applications, Academic press, London, 1999, pp. 165–189 (https://doi.org/10.1016/B978-0-12-598920-6.X5000-3)
M. M. Dubinin, J. Colloid Interface Sci. 23 (1967) 487 (https://doi.org/10.1016/0021-9797(67)90195-6)
E. P. Barrett, L. G. Joyner, P. P. Halenda, J. Am. Chem. Soc. 73 (1951) 373 (https://doi.org/10.1021/ja01145a126)
I. Langmuir, J. Am. Chem. Soc. 40 (1918)1361 (https://doi.org/10.1021/ja02242a004)
H. M. F. Freundlich, Z. Phys. Chem. A 57 (1906) 385 (https://doi.org/10.1515/zpch-1907-5723)
S. Lagergren, Handlingar 24 (1898) 1 (https://doi.org/10.1002/andp.18983000208)
Y. S. Ho, J. C. Y. Ng, G. McKay, Sep. Purif. Meth. 29 (2000) 189 (https://doi.org/10.1081/SPM-100100009)
A. Ivanovska, L. Pavun, B. Dojčinović, M. Kostić, J. Serb. Chem. Soc. 86 (2021) 885 (https://doi.org/10.2298/JSC210209030I)
F. Rodriguez-Reinoso, M. Molina-Sabio, Coloids Surfaces, A 241 (2004) 15 (https://doi.org/10.1016/j.colsurfa.2004.04.007)
Q. Qian, M. Machida, H. Tatsumoto, Bioresour. Technol. 98 (2007) 353 (https://doi.org/10.1016/j.biortech.2005.12.023)
29. S. Yorgun, N. Vural, H. Demiral, Micropor. Mesopor. Mater. 122 (2009) 189 (https://doi.org/10.1016/j.micromeso.2009.02.032)
A. C. Lua, T. Yang, J. Colloid Interf. Sci. 290 (2005)505 (https://doi.org/10.1016/j.jcis.2005.04.063)
M. M. Gómez-Tamayo, A. Macías-García, M. A. Díez, E. M. Cuerda-Correa, J. Hazard. Mater. 153 (2008) 28 (https://doi.org/10.1016/j.jhazmat.2007.08.012)
K. Mohanty, D. Das, M. N. Biswas, Adsorption 12 (2006) 119 (https://doi.org/10.1007/s10450-006-0374-2)
J. Yang, K. Qiu, Chem. Eng. J. 167 (2011) 148 (https://doi.org/10.1016/j.cej.2010.12.013)
I. Herath, P. Kumarathilaka, M. I. Al-Wabel, A. Abduljabbar, M. Ahmad, A. R. A. Usman, M. Vithanage, Micropor. Mesopor. Mater. 225 (2016) 280 (https://doi.org/10.1016/j.micromeso.2016.01.017)
B. H. Hameed, R. R. Krishni, S. A. Sata, J. Hazard. Mater. 162 (2009) 305 (https://doi.org/10.1016/j.jhazmat.2008.05.036)
M. M. Nourouzi, T. G. Chuah, T. S. Y. Choong, Desalin. Water Treat. 24 (2010) 321 (https://doi.org/10.5004/dwt.2010.1461)
K. Sen, J. K. Datta, N. K. Mondal, Appl. Water Sci. 9 (2019) 162 (https://doi.org/10.1007/s13201-019-1036-3)
D. C. Nguyena, A. I. Vezentseva, P. V. Sokolovskiyc, A. A. Greishc, Russ. J. Phys. Chem., A 95 (2021) 1212 (https://doi.org/10.1134/S0036024421060194)
Q. Yang, J. Wang, X. Chen, W. Yang, H. Pei, N. Hu, Y. Li, Y. Suo, T. Lic, J. Wang, J. Mater. Chem. 6 (2018) 2184 (https://doi.org/10.1039/C7TA08399H)
F. Chen, C. Zhou, G. Li, F. Peng, Arab. J. Chem. 9 (2016) S1665 (https://doi.org/10.1016/j.arabjc.2012.04.014).