Synthesis and antiproliferative activity of new thiazole hybrids with [3.3.0]furofuranone or tetrahydrofuran scaffolds Scientific paper
Main Article Content
Abstract
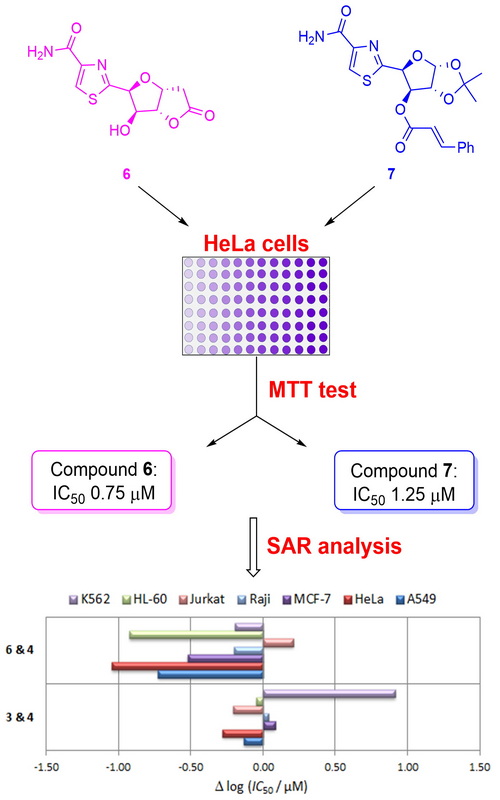
New thiazole hybrids were synthesized and evaluated for their in vitro cytotoxicity against a panel of human malignant cell lines. The key steps in the synthesis of hybrids 3–7 involved the initial condensation of appropriate aldononitriles with cysteine ethyl ester hydrochloride, followed by subsequent treatment of resulting thiazolines with diazabicycloundecene to form the thiazole ring. Bioisosteres 8 and 14 have been prepared after the stereoselective addition of 2-(trimethylsilyl)thiazole to the hemiacetals obtained by periodate cleavage of terminal diol functionality in the suitably protected d-glucose derivatives. The obtained analogues showed various antiproliferative activities in the cultures of several tumour cell lines. Hybrid 6 was the most potent in HeLa cells, exhibiting more than 10 and 4 times stronger activity than both leads 1 and 2, respectively. The most active compound in Raji cells was hybrid 12, which was nearly 2-fold more potent than the clinical antitumour drug doxorubicin. All analogues were more potent in A549 cells with respect to lead 1, while compounds 6 and 7 were slightly more active than doxorubicin. Preliminary structure–activity relationship analysis revealed that the presence of a cinnamate group at the C-3 position in analogues of type 7 increases the activity of resulting molecular hybrids.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja,Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-68/2020-14/200125 -
Serbian Academy of Sciences and Arts
Grant numbers F-130
References
H. M. Sampath Kumar, L. Herrmann, S. B. Tsogoeva, Bioorg. Med. Chem. Lett. 30 (2020) 127514 (https://doi.org/10.1016/j.bmcl.2020.127514)
V. Ivasiv, C. Albertini, A. E. Gonçalves, M. Rossi, M. L. Bolognesi, Curr. Top. Med. Chem. 19 (2019) 1694 (https://doi.org/10.2174/1568026619666190619115735)
C. Viegas-Junior, A. Danuello, V. da Silva Bolzani, E. J. Barreiro, C. A. Manssour Fraga, Curr. Med. Chem. 14 (2007) 1829 (https://doi.org/10.2174/092986707781058805)
А. Petrou, M. Fesatidou, A. Geronikaki, Molecules 26 (2021) 3166 (https://doi.org/10.3390/molecules26113166)
D. S. Bhagat, P. A. Chawla, W. B. Gurnule, S. K. Shejul, G. S. Bumbrah, Curr. Org. Chem. 25 (2021) 819 (https://doi.org/10.2174/1385272825999210101234704)
M. Svirčev, M. Popsavin, A. Pavić, B. Vasiljević, M. V. Rodić, S. Djokić, J. Kesić, B. Srećo Zelenović, V. Popsavin, V. Kojić, Bioorg. Chem. 121 (2022) 105691 (https://doi.org/10.1016/j.bioorg.2022.105691)
X.-S. Peng, R. M. P. Ylagan, Y. M. Siu, H. N. C. Wong, Chem. Asian J. 10 (2015) 2070 (https://doi.org/10.1002/asia.201500288)
X. Fang, J. E. Anderson, C. Chang, P. E. Fanwick, J. L. McLaughlin, J. Chem. Soc. Perkin Trans. I (1990) 1655 (https://doi.org/10.1039/P19900001655)
K. Malek, M. S. Boosalis, K. Waraska, B. S. Mitchell, D. G. Wright, Leukemia Res. 28 (2004) 1125 (https://doi.org/10.1016/j.leukres.2004.03.003)
D. G. Wright, M. Boosalis, K. Malek, K. Waraska, Leukemia Res. 28 (2004) 1137 (https://dx.doi.org/10.1016/j.leukres.2004.03.004)
А. P. Rauter, J. A. Figueiredo, I. M. Ismael, Carbohydr. Res. 188 (1989) 19 (https://dx.doi.org/10.1016/0008-6215(89)84054-6)
P. Köll, A. Wernicke, J. Kovács, A. Lützen, J. Carbohydr. Chem. 19 (2000) 1019 (http://dx.doi.org/10.1080/07328300008544132)
D. A. Scudiero, R. H. Shoemaker, K. D. Paull, A. Monks, S. Tierney, T. H. Nofziger, M. J. Currens, D. Seniff, M. R. Boyd, Cancer Res. 48 (1988) 4827 (https://cancerres.aacrjournals.org/content/48/17/4827)
I. Kovačević, M. Popsavin, G. Benedeković, J. Kesić, V. Kojić, D. Jakimov, T. Srdić-Rajić, G. Bogdanović, V. Divjaković, V. Popsavin, Eur. J. Med. Chem. 134 (2017) 293 (http://dx.doi.org/10.1016/j.ejmech.2017.03.088)
S. Valverde, M. Martin-Lomas, B. Herradon, S. Garcia-Ochoa, Tetrahedron 43 (1987) 1895 (https://doi.org/10.1016/S0040-4020(01)81502-7)
B. Neises, W. Steglich, Angew. Chem. Int. Ed. Engl. 17 (1978) 522 (https://doi.org/10.1002/anie.197805221)
P. De, M. Baltas, F. Bedos-Belval, Curr. Med. Chem. 18 (2011) 1672 (https://doi.org/10.2174/092986711795471347)
E. Pontiki, A. Peperidou, I. Fotopoulos, D. Hadjipavlou-Litina, Curr. Pharm. Biotechnol. 19 (2018) 1019 (https://doi.org/10.2174/1389201019666181112102702)
L.‐S. Feng, J.‐B. Cheng, W.‐Q. Su, H.‐Z. Li, T. Xiao, D.‐A. Chen, Z.‐L. Zhang, Arch. Pharm. 355 (2022) 2200052 (https://doi.org/10.1002/ardp.202200052)
G. Benedeković, J. Francuz, I. Kovačević, M. Popsavin, B. Srećo Zelenović, V. Kojić, G. Bogdanović, V. Divjaković, V. Popsavin, Eur. J. Med. Chem. 82 (2014) 449 (http://dx.doi.org/10.1016/j.ejmech.2014.05.081)
M. Svirčev, G. Benedeković, I. Kovačević, M. Popsavin, V. Kojić, D. Jakimov, T. Srdić-Rajić, M. V. Rodić, V. Popsavin, Tetrahedron 74 (2018) 4761 (https://doi.org/10.1016/j.tet.2018.07.046)
А. Dondoni, G. Fantin, M. Fogagnolo, A. Medici, Tetrahedron 43 (1987) 3533 (https://doi.org/10.1016/S0040-4020(01)81646-X)
T. D. Inch, Carbohydr. Res. 5 (1967) 53 (https://doi.org/10.1016/0008-6215(67)85007-9)
C. R. Groom, I. J. Bruno, M. P. Lightfoot, S. C. Ward, Acta Crystallogr., B 72 (2016) 171 (https://doi.org/10.1107/S2052520616003954)
M. Sundaralingam, Biopolymers 7 (1969) 821 (https://doi.org/10.1002/bip.1969.360070602)
W. Seanger, Principles of Nucleic Acid Structure, Springer-Verlag, New York, 1984
D. Cremer, J. A. Pople, J. Am. Chem. Soc. 97 (1975) 1354 (https://doi.org/10.1021/ja00839a011)
C. Altona, M. Sundaralingam, J. Am. Chem. Soc. 94 (1972) 8205 (https://doi.org/10.1021/ja00778a043)
H. P. M. de Leeuw, C. A. G. Hasnoot, C. Altona, Isr. J. Chem. 20, (1980) 108 (https://doi.org/10.1002/ijch.198000059)
А. Dondoni, M.-C. Scherrmann, J. Org. Chem. 59 (1994) 6404 (https://doi.org/10.1021/jo00100a050).