In vitro study of redox properties of azolyl-lactones in human serum Scientific paper
Main Article Content
Abstract
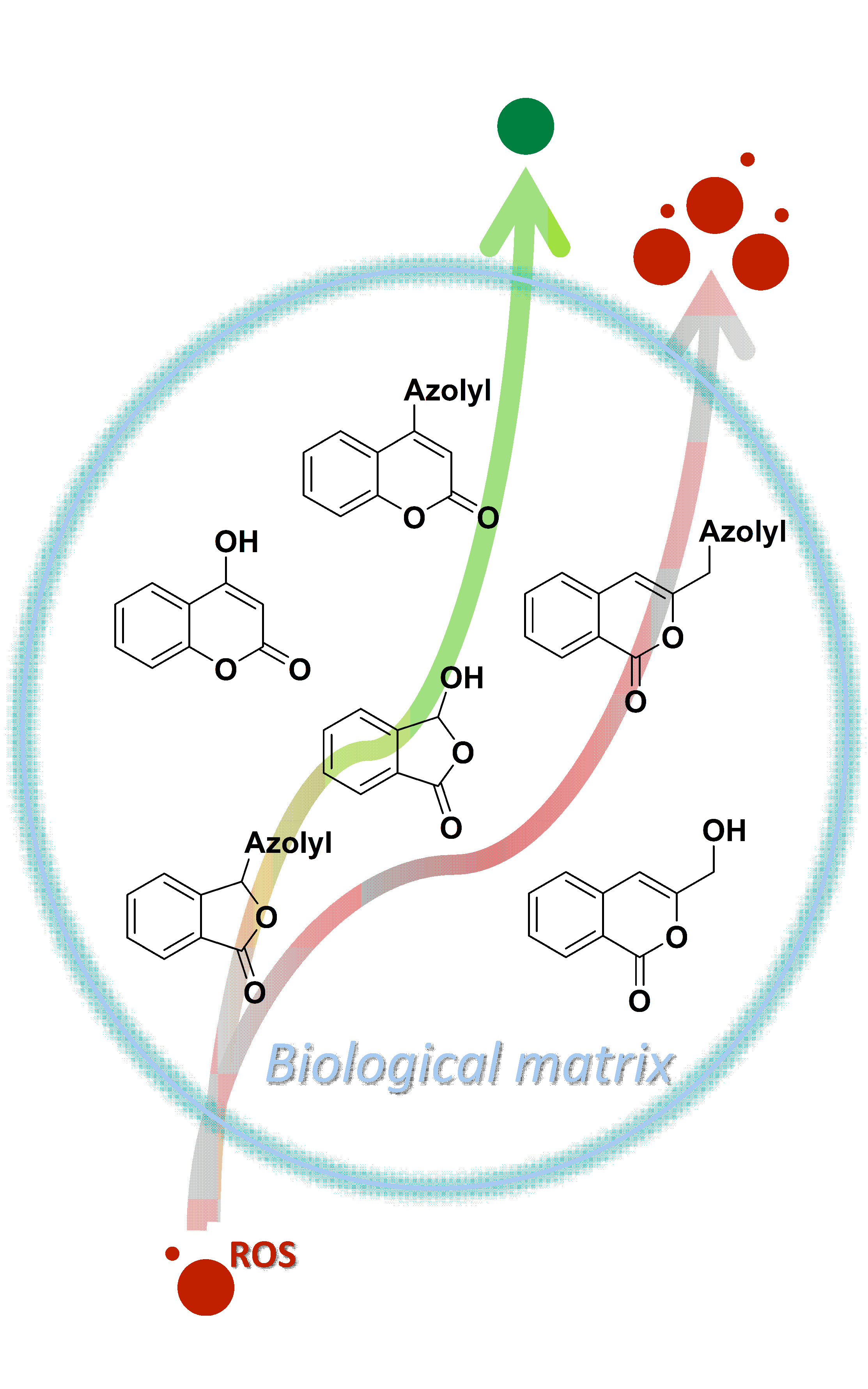
Disruption of the redox balance in the body causes oxidative stress that can initiate many diseases. While antioxidants reduce the level of oxidizing compounds in the medium, prooxidants promote the opposite process and have been used in therapies in particular those of cancer diseases. In this study, a series of azolyl lactones, were tested in human serum as a biological matrix and the obtained values of their oxy scores (OS) were compared. The antioxidative properties of these compounds were also tested under conditions of induced oxidative stress using an external prooxidant, t-butylhydroperoxide. The results showed that the sulphur analogue 4-azolyl coumarin 5 has the best antioxidant properties (OS –2.2), while the halogenated derivatives of pyrazolylcoumarin 7 and 8 act as prooxidants, but successfully resist oxidative stress (OS 2.7 and 2.0, respectively). Related phthalides and isocoumarins showed prooxidative properties, but azolyl isocoumarins 10 and 11 show the strongest resistance to oxidative stress, reflected in their negative oxy score value (OS –2.1 and –1.1, respectively). The results demonstrated that combining two pharmacophores with known redox properties can produce potent compounds in both directions, with the antioxidative and the prooxidative characteristics.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja,Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-68/2022-14/200161
References
C. H. Foyer, G. Noctor, The Plant Cell 17 (2005) 1866 (https://doi.org/10.1105/tpc.105.033589)
H. Sies, Antioxidants 9 (2020) 852 (https://doi.org/10.3390/antiox9090852)
I. Liguori, G. Russo, F. Curcio, G. Bulli, L. Aran, D. Della-Morte, G. Gargiulo, G. Testa, F. Cacciatore, D. Bonaduce, P. Abete, Clin. Interv. Aging 13 (2018) 757 (https://doi.org/10.2147/CIA.S158513)
J. D. Hayes, A. T. Dinkova-Kostova, K. D. Tew, Cancer Cell 38 (2020) 167 (https://doi.org/10.1016/j.ccell.2020.06.001)
M. Mihajlovic, B. Ivkovic, B. Jancic-Stojanovic, A. Zeljkovic, V. Spasojevic-
-Kalimanovska, J. Kotur-Stevuljevic, D. Vujanovic, Anticancer Drugs 31 (2020) 942 (https://doi.org/10.1097/cad.0000000000000924)
I. Kostova, S. Bhatia, P. Grigorov, S. Balkansky, V. S. Parmar, A. K. Prasad, L. Saso, Curr. Med. Chem. 18 (2011) 3929 (https://doi.org/10.2174/092986711803414395)
Z. Rehakova, V. Koleckar, L. Jahodar, L. Opletal, K. Macakova, L. Cahlikova, D. Jun, K. Kuca, J. Enzyme Inhib. Med. Chem. 29 (2014) 49 (https://doi.org/10.3109/14756366.2012.753589)
G. B. Bubols, D. da R. Vianna, A. Medina-Remon, G. von Poser, R. M. Lamuela-Raventos, V. L. Eifler-Lima, S. C. Garcia, Mini Rev. Med. Chem. 13 (2013) 318 (https://doi.org/10.2174/138955713804999775)
D. Procházková, I. Boušová, N. Wilhelmová, Fitoterapia 82 (2011) 513 (https://doi.org/10.1016/j.fitote.2011.01.018)
M. Sökmen, M. Akram Khan, Inflammopharmacol 24 (2016) 81 (https://doi.org/10.1007/s10787-016-0264-5)
K. Tianpanich, S. Prachya,S. Wiyakrutta, C. Mahidol, S. Ruchirawat, P. Kittakoop, J. Nat Prod 74 (2011) 79 (https://doi.org/10.1021/np1003752)
M. Frombaum, S. Le Clanche, D. Bonnefont-Rousselot, D. Borderie, Biochimie 94 (2012) 269 (https://doi.org/10.1021/np1003752)
T. Janković, N. Turković, J. Kotur-Stevuljević, Z. Vujić, B. Ivković, Chem. Biol. Interact. 324 (2020) 109084 (https://doi.org/10.1016/j.cbi.2020.109084)
X. Qiang, Y. Li, X. Yang, L. Luo, R. Xu, Y. Zheng, Z. Cao, Z. Tan, Y. Deng, Bioorganic & Med Chem Lett 27 (2017) 718 (https://doi.org/10.1016/j.bmcl.2017.01.050)
W. S. Hamama, M. A. Berghot, E. A. Baz, M. A. Gouda, Arch. Pharm. 344 (2011) 710 (https://doi.org/10.1002/ardp.201000263)
A. A. Al-Amiery, Y. K. Al-Majedy, A. A. H. Kadhum, A. B. Mohamad, PLOS ONE 10 (2015) e0132175 (https://doi.org/10.1371/journal.pone.0132175)
E. Y. Ahmed, O. M. Abdelhafez, D. Zaafar, A. M. Serry, Y. H. Ahmed, R. F. A. El-Telbany, Z. Y. Abd Elmageed, H. I. Ali, Arch. Pharm. 355 (2022) 2100327 (https://doi.org/10.1002/ardp.202100327)
I. A. M. Radini, D. A. Ibrahim, R. E. Khidre, Acta Pol. Pharm. 79 (2019) 453 (https://doi.org/10.32383/appdr/102651)
M. R. Simić, S. Erić, I. Borić, A. Lubelska, G. Latacz, K. Kiec-Kononowicz, S. Vojnović, J. Nikodinović-Runić, V. M. Savić, J. Serb. Chem. Soc. 86 (2021) 639 (https://doi.org/10.2298/JSC201201025S)
M. Simic, M. Petkovic, P. Jovanovic, M. Jovanovic, G. Tasic, I. Besu, Z. Zizak, I. Aleksic, J. Nikodinovic-Runic, V. Savic, Arch. Pharm. 354 (2021) 2100238 (https://doi.org/10.1002/ardp.202100238)
K. G. Guimarães, R. P. de Freitas, A. L. T. G. Ruiz, G. F. Fiorito, J. E. de Carvalho, E. F. F. da Cunha, T. C. Ramalho, R. B. Alves, Eur. J. Med. Chem. 111 (2016) 103 (https://www.sciencedirect.com/science/article/pii/S0223523416300599)
O. Erel, Clin. Biochem. 38 (2005) 1103 (https://doi.org/10.1016/j.clinbiochem.2005.08.008)
J. Kotur-Stevuljevic, N. Bogavac-Stanojevic, Z. Jelic-Ivanovic, A. Stefanovic, T. Gojkovic, J. Joksic, M. Sopic, B. Gulan, J. Janac, S. Milosevic, Atherosclerosis 241 (2015) 192 (https://doi.org/10.1016/j.atherosclerosis.2015.05.016)
O. Erel, Clin. Biochem. 37 (2004) 277 (https://doi.org/10.1016/j.clinbiochem.2003.11.015)
D. H. Alamdari, K. Paletas, T. Pegiou, M. Sarigianni, C. Befani, G. Koliakos, Clin. Biochem. 40 (2007) 248 (https://doi.org/10.1016/j.clinbiochem.2006.10.017)
G. L. Ellman, Arch. Biochem. Biophys. 82 (1959) 70 (https://doi.org/10.1016/0003-9861(59)90090-6)
E. Taylan, H. Resmi, Turkish J. Biochem. 35 (2010) 275 (https://web.citius.technology/upload/turkjbiochem/2010/275-278.pdf)
C. K.Riener, G. Kada, H.J. Gruber, Anal. Bioanal. Chem, 373 (2002) 266 (https://doi.org/10.1007/s00216-002-1347-2)
D. Crich, L. Quintero, Chem. Rev. 89 (1989) 1413 (https://doi.org/10.1021/cr00097a001)
N. Ivanović, L. Jovanović, Z. Marković, V. Marković, M. D. Joksović, D. Milenković, P. T. Djurdjević, A. Ćirić, L. Joksović, ChemistrySelect 1 (2016) 3870 (https://doi.org/10.1002/slct.201600738)
F. Denés, M. Pichowicz, G. Povie, P. Renaud, Chem. Rev. 114 (2014) 2587 (https://doi.org/10.1021/cr400441m)
Q. Cai, G. Takemura, M. Ashraf, J. Cardiovasc. Pharmacol. 25 (1995) 147 (https://doi.org/10.1097/00005344-199501000-00023)
S. Puntarulo, A. I. Cederbaum, Arch. Biochem. Biophys. 255 (1987) 217 (https://doi.org/10.1016/0003-9861(87)90388-2)
L. F. da Cruz, C. G. Santos, T. P. R. Gonçalves, G. D. Marena, I. L. A. Souza, L. A. R. dos S. Lima, F. M. D. Chequer, F. C. H. Pinto, F. A. R. Nogueira, M. G. de F. Araujo, Res. Soc. Dev. 10 (2021) e7910816948 (https://doi.org/10.33448/rsd-v10i8.16948)
E. H. Avdović, I. P. Petrović, M. J. Stevanović, L. Saso, J. M. Dimitrić Marković, N. D. Filipović, M. Ž. Živić, T. N. Cvetić Antić, M. V. Žižić, N. V. Todorović, M. Vukić, S. R. Trifunović, Z. S. Marković, Oxid. Med. Cell. Longev. 2021 (2021) e8849568 (https://doi.org/10.1155/2023/9979397)
M. Tanaka, S. Motomiya, A. Fujisawa, Y. Yamamoto, J. Clin. Biochem. Nutr. 61 (2017) 164 (https://doi.org/10.3164/jcbn.17-75)
L. A. Clejan, A. I. Cederbaum, Biochim. Biophys. Acta Gen. Subj. 1034 (1990) 233 (https://doi.org/10.1016/0304-4165(90)90082-8).