Response surface methodology for the study of interactions between components in a micellar system formulation Scientific paper
Main Article Content
Abstract
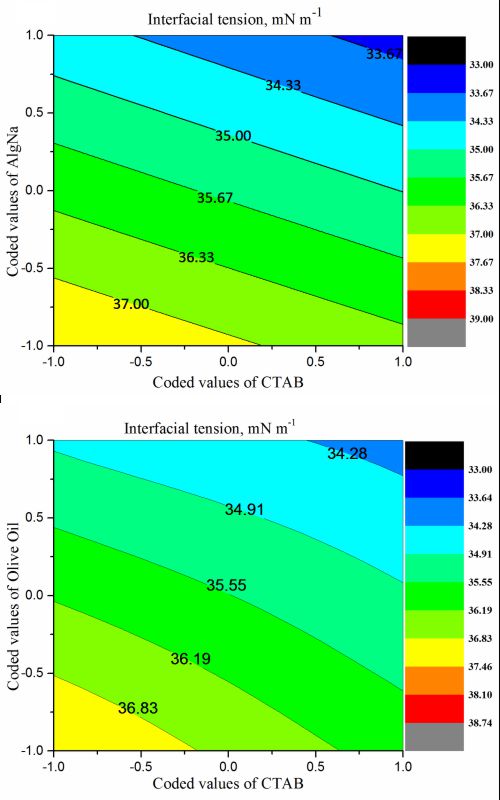
This work was aimed at the examination of the interaction of certain physicochemical properties on micellar systems constituting of a polymer (sodium alginate), two surfactants (CTAB and tween 80), and Algerian olive oil. Response surface modelling (RSM) was applied to study the combined effects of systems containing each type of surfactant. The monitoring of four independent parameters, i.e., the interfacial tension (Y1), the conductivity (Y2), the viscosity (Y3) and the turbidity (Y4) as responses for the experimental design model, allowed the determination of the performance of the established models. Based on statistical analyzes, the coefficients R2 and Q2 for the interfacial tension, the conductivity, the viscosity and the turbidity are: 0.998 and 0.805; 0.982 and 0.742; 0.976 and 0.734, and 0.985 and 0.723, respectively. The obtained results indicate that these models showed a good predictive power for an optimal system composed of CTAB, Tween 80, AlgNa, and olive oil. For the CTAB/AlgNa and CTAB/olive oil systems, interfacial tension values of 33.85 and 34.39 mN m-1, respectively, and maximum conductivity values of 4.126 and 4.064 mScm-1, respectively, were obtained. For viscous compounds consisting of AlgNa/Olive Oil and AlgNa/Tween 80, maximum viscosity values of 202.5 and 196.6 mPa s, respectively, were obtained. For the same systems as those for viscosity, turbidity values of 300 and 304 NTU, respectively, were obtained.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
A. Avarnas, I. Panagiotis, J. Colloid Interface Sci. 258 (2003) 102 (https://doi.org/10.1016/S0021-9797(02)00129-7)
N. Kamenka, I. Burgaud, C. Treiner, R. Zana, Langmuir 10(1994) 3455 (https://doi.org/10.1021/la00022a016)
L. M. Smitter, J. Guédez, A. J. Müller, J. Colloid Interface Sci. 236 (2001) 343 (https://doi.org/10.1006/jcis.2001.7438)
T. Gilanyl, E. Wolfram, Colloids Surfaces 3 (1981) 181 (https://doi.org/10.1016/0166-6622(81)80077-7)
R. Barreiro-Iglesias, C. Alvarez-Lorenzo, A. Concheiro, Int. J. Pharm. 258 (2003) 165 (https://doi: 10.1016/s0378-5173(03)00182-0)
E. Minatti, D. Zanette, Colloids Surfaces, A 113 (1996) 237 (http://dx.doi.org/10.1016/0927-7757(96)03573-X)
T. Casgrove, S. J. Mears, T. Obey, L. Thompson, R. D. Welsey, Colloids Surfaces, A 149 (1999) 329 (https://doi.org/10.1016/S0927-7757(98)00301-X)
W. Guo, Y.W. Sun, G.S. Luo, Y.J. Wang, Colloids Surfaces, A 252 (2005) 71 (https://doi.org/10.1016/j.colsurfa.2004.10.013)
S. Puvvada, D. Blankschtein, J. Chem. Phys. 92 (1990) 371 (https://doi.org/10.1063/1.457829)
K. Holmberg, B. Jönsson , B. Kronberg , B. Lindman, Surfactants and polymers in aqueous solution, 2nd ed., John Wiley & Sons, LTD, New York, 2002 (ISBN: 0-471-49883-1)
A.I. Khuri, J.A. Cornell, Response surfaces, design and analysis, Marcel Dekker Inc., New York,1996, p. 536 (ISBN 978-0-367-40125-2)
D. C. Montgomery, Design and analysis of experiments, 3rd ed., John Willey & Sons Inc., New York, 1991, p. 688 (ISBN 978-1-119-49244-3)
K. G. S. Nair, R. Velmurugan, S. K. Sukumaran, Bio. Nano Sci. 10 (2020) 279 https://doi.org/10.1007/s12668-019-00713-0
J. Goupy, Anal. Chim. Acta 544 (2005) 184 (https://doi.org/10.1016/j.aca.2005.01.051)
X. Huang, P. Jiang, Adv. Mater. 27 (2005) 546 https://doi.org/10.1002/adma.201401310
W. G. Cochran, G. M. Cox, Experimental designs, 2nd ed., John Willey & Sons Inc., New York, 1990, p. 335 (ISBN978-0-471-54567-5)
M. Nedjhioui, J. P. Canselier, N. Moulai Mostefa, A. Bensmaili, A. Skender, Desalination 206 (2007) 589 (https://doi.org/10.1016/j.desal.2006.04.065)
M. Nedjhioui, N. Moulai Mostefa, A. Bensmaili, A. Morsli, Desalination 185 (2005) 543 (https://doi.org/10.1016/j.desal.2005.05.013)
M. Nedjhioui, J. P. Canselier, N. Moulai-Mostefa, A. Bensmaili, J. Disper. Sci. Technol. 30 (2009) 1331 (https://doi.org/10.1080/01932690902735538)
M. Nedjhioui, N. Moulai-Mostefa, A. Sellami, F.Toubal, Desalin.Water. Тreat. 56 (2015) 2739 (https://doi.org/10.1080/19443994.2015.1012339)
M. Nedjhioui, N. Moulai-Mostefa, M. Tir, Desalin. Water. Treat. 55 (2015) 3704 (https://doi.org/10.1080/19443994.2014.940217)
N. Moulai-Mostefa, R. Khalladi, M. Nedjhioui, Ann. Chim. Sci. Mat. 32 (2007) 421 (https://doi: 10.3166/acsm.32.421-429)
A. Dal Bo, B. Schweitzer, A. C. Felippe, D. Zanette, B. Lindman, Colloids Surfaces, A 256 (2005) 171 (https://doi.org/10.1016/j.colsurfa.2005.01.017)
T. D. Blake, Y. D. Shikhmurzaev, J. Colloid Interface Sci. 253 (2002) 196 (https://doi.org/10.1006/jcis.2002.8513)
D. Langevin, Adv. Colloid Interface Sci. 89 (2001) 467 (https://doi.org/10.1016/S0001-8686(00)00068-3)
E. D. Goddard, Colloids Surfaces 19 (1986) 255 (https://doi.org/10.1016/0166-6622(86)80340-7)
V. J. Sovilj, L. B. Petrovic, Carbohyd.Polym. 64 (2006) 41 (https://doi.org/10.1016/j.carbpol.2005.10.030)
I. M. Harrison, F. Candau, R. Zana, Colloid Polym. Sci. 277 1 (1999) 48 (https://doi.org/10.1007/s003960050366)
J. Merta, P. Stenius, E. Pirttinen, J. Disper. Sci. Technol. 20 (1999) 677 (https://doi.org/10.1080/01932699908943814).