Crystal structure of K3EuSi2O7 Scientific paper
Main Article Content
Abstract
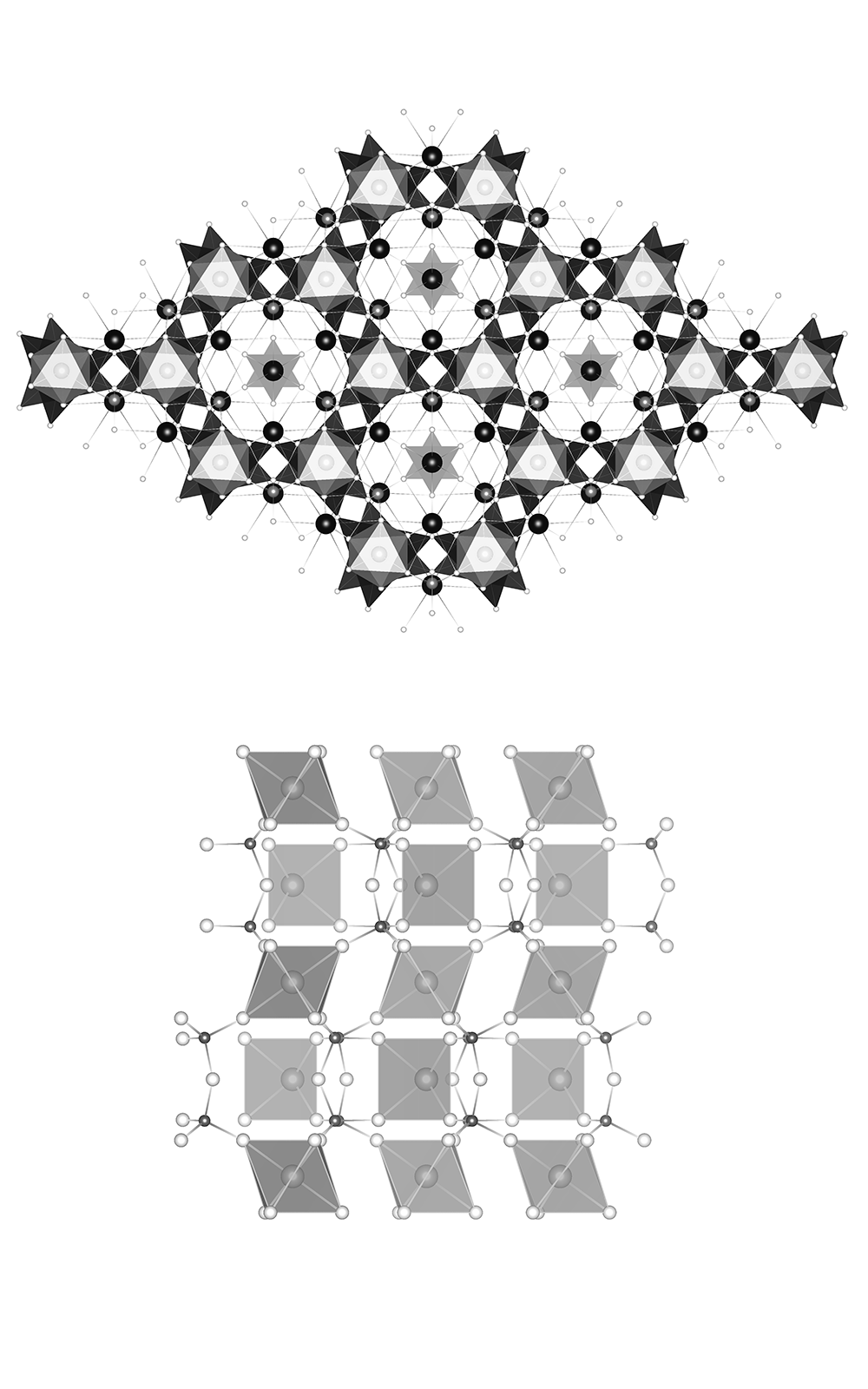
As part of research on the flux technique for growing alkali rare-earth elements (REE) containing silicates, tripotassium europium disilicate, K3EuSi2O7, was synthesized and characterized by single-crystal X-ray diffraction. It crystallizes in the space group P63/mcm. In the crystal structure of the title compound, one part of the Eu cations are in a slightly distorted octahedral coordination and the other part are in an ideal trigonal prismatic coordination environment. The disilicate Si2O7 groups connect four EuO6 octahedra and one EuO6 trigonal prism. Three differently coordinated potassium cations are located between them. Silicates containing the larger rare earth elements usually crystallize in a structure that contains the rare-earth cation in both a slightly distorted octahedral and an ideal trigonal prismatic coordination environment.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
J. Felsche, In The Crystal Chemistry of the Rare-Earth Silicates. Rare Earths. Structure and Bonding, Vol. 13, Springer, Heidelberg, 1973 (https://doi.org/10.1007/3-540-06125-8_3)
A. Kitai, In Luminescent Materials and Applications, John Wiley & Sons Ltd., Chichester, 2008 (Online ISBN:9780470985687)
A. M. Latshaw, W. M. Chance, N. Trenor, G. Morrison, M. D. Smith, J. Yeon, D. E. Williams, H.-C. zur Loye, CrystEngComm 17 (2015) 4691 (https://doi.org/10.1039/C5CE00630A)
A. M. Latshaw, K. D. Hughey, M. D. Smith, J. Yeon, H.-C. zur Loye, Inorg. Chem. 54 (2015) 876 (https://doi.org/10.1021/ic502185b)
A. M. Latshaw, B. O. Wilkins, K. D. Hughey, J. Yeon, D. E. Williams, T. T. Tran, P. S. Halasyamani, H.-C. zur Loye, CrystEngComm 17 (2015) 4654 (https://doi.org/10.1039/C5CE00671F)
A. M. Latshaw, G. Morrison, K. D. zur Loye, A. R. Myers, M. D. Smith, H.-C. zur Loye, CrystEngComm 18 (2016) 2294 (https://doi.org/10.1039/C6CE00177G)
A. M. Latshaw, J. Yeon, M. D. Smith, H.-C. zur Loye, J. Solid State Chem. 235 (2016) 100 (https://doi.org/10.1016/j.jssc.2015.12.013)
G. Morrison, A. M. Latshaw, N. R. Spagnuolo, H.-C. Zur Loye, J. Am. Chem. Soc. 139 (2017) 14743 (https://doi.org/10.1021/jacs.7b08559)
B. R. Figueiredo, A. A. Valente, Z. Lin, C. M. Silva, Micropor. Mesopor. Mat. 234 (2016) 73 (https://doi.org/10.1016/j.micromeso.2016.07.004)
F. Liebau, Structural chemistry of silicates: structure, bonding and classification, Springer, Heidelberg , 1985, p. 347 (https://doi.org/10.1007/978-3-642-50076-3)
I. A. Bondar, T. F. Tenisheva, Y. F. Shepelev, N. A. Toropov, Dokl. Akad. Nauk SSSR 160 (1965) 1069 (http://www.mathnet.ru/links/df924f2db1305a2430f200e3f58341c7/dan30741.pdf)
M. S. Hwang, H. Y.-P. Hong, M. C. Cheng, Y. Wang, Acta Cryst., C 43 (1987) 1241 (https://doi.org/10.1107/S0108270187092308)
I. Vidican, M. Smith, M., H.-C. zur Loye, J. Solid State Chem. 170 (2003) 203 (https://doi.org/10.1016/S0022-4596(02)00029-4)
J. D. Napper, R. C. Layland, M. D. Smith, H. Loye, J. Chem. Crystallogr. 34 (2004) 347 (https://doi.org/10.1023/B:JOCC.0000028666.53348.fc)
P. Dabić, M. G. Nikolić, S. Kovač, A. Kremenović, Acta Cryst., C 75 (2019) 1417 (https://doi.org/10.1107/S2053229619011926)
A. Myers, J. South Carolina Acad. Sci. 12 (2014) 200 (https://scholarcommons.sc.edu/jscas/vol12/iss1/1)
Rigaku Oxford Diffraction, CrysAlisPro Software system, Rigaku Corporation, Oxford, 2018
Rigaku PDXL 2: Integrated powder X-ray diffraction software. Version 2.8.3.0, Rigaku Corporation, Tokyo, 2007 https://www.rigaku.com/en/service/software/pdxl
R. C. Clark, J. S. Reid, Acta Cryst., A 51 (1995) 887 (https://doi.org/10.1107/S0108767395007367)
G. M. Sheldrick, Acta Cryst., C 71 (2015) 3 (https://doi.org/10.1107/S2053229614024218)
M. Momma, F. Izumi, J. Appl. Cryst. 44 (2011) 1272 (https://doi.org/10.1107/S0021889811038970)
P. Dabić, V. Kahlenberg, B. Krueger, M. Rodić, S. Kovač, J. Blanuša, Z. Jagličić, Lj. Karanović, V. Petríček, A. Kremenović, Acta Cryst., B, under review
A. S. Wills, VaList, 2010, Program available from www.CCP14.ac.uk
I. D. Brown, D. Altermatt, Acta Cryst., B 41 (1985) 244 (https://doi.org/10.1107/S0108768185002063)
N. E. Brese, M. OKeeffe, Acta Cryst., B 47 (1991) 192 (https://doi.org/10.1107/S0108768190011041)
R. D. Shannon, Acta Cryst., A 32 (1976) 751 (https://doi.org/10.1107/S0567739476001551).