Aspirin–hydrogel ocular film for topical delivery and ophthalmic anti-inflammation Scientific paper
Main Article Content
Abstract
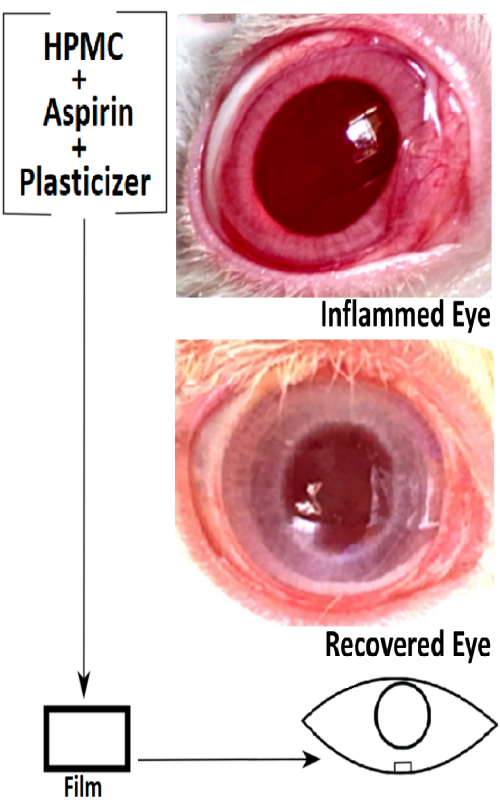
Ocular drug delivery in hydrogel forming film form has several benefits over conventional dosage forms. An ophthalmic anti-inflammation study was undertaken using topically applied aspirin in a hydrogel film formulation. A hydroxypropyl methylcellulose (HPMC) matrix film formulation was prepared by the solvent casting and evaporation technique by taking triethanolamine (TEA) as a plasticizer. Ex vivo corneal permeation as well as anti-inflammatory potential of aspirin was studied on carrageenan induced rabbit eye model. Moisture uptake was found to be in the range of 17.1 and 19.1 % for all the film formulations. The film with the higher HPMC content exhibited both increased moisture uptake and amount of swelling. Among the formulations, the swelling order was found to increase with increasing amount of HPMC in the film. Presence of the hydrogel matrix forming polymer sustained the drug release and corneal permeation for more than 6 h and controlled the process by the diffusion mechanism. The signs of carrageenan induced acute inflammation was inhibited completely within just 2 h of placing the film in the rabbit eye whilst the positive control continued showing redness and increased tear secretion. Aspirin ocular film formulation could be utilized for ocular anti-inflammation for an extended period of time with better patient compliance.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
G. Fang, Q. Wang, X. Yang, Y. Qian, G. Zhang, Q. Zhu, B. Tang, Colloids Surfaces,. A 627 (2021) 127187 (https://doi.org/10.1016/j.colsurfa.2021.127187)
G. Fan, G. Li-li, W. Yan-rong, Q.I. Wang, Int. Eye Sci. 17 (2017) 2359 (https://doi.org/10.3980/j.issn.1672-5123.2017.12.45)
M. T. Kralinger, M. Voigt, G. F. Kieselbach, D. Hamasaki, B. C. Hayden, J. M. Parel, Ophthalmic Res. 35 (2003) 102 (https://doi.org/10.1159/000069129)
S. Das, J. R. Bellare, R. Banerjee, Colloids Surfaces, B 93 (2012) 161 (https://doi.org/10.1016/j.colsurfb.2011.12.033)
A. Yazici, E. Sarı, E. Ayhan, J. Ocul. Pharmacol. Ther. 34 (2018) 256 (https://doi.org/10.1089/jop.2017.0064)
Ameeduzzafar, J. Ali, M. Fazil, M. Qumbar, N. Khan, A. Ali, Drug Deliv. 23 (2016) 700 (https://doi.org/10.3109/10717544.2014.923065)
L. Battaglia, M. Gallarate, L. Serpe, F. Foglietta, E. Muntoni, A. P. Rodriguez, M. Angeles, S. Aspiazu, in Lipid Nanocarriers for Drug Targeting, A. M. Grumezescu, Ed., William Andrew Applied Science Publishers, Norwich, NY, 2018, pp. 269–312 (https://doi.org/10.1016/B978-0-12-813687-4.00007-4)
A. Pramanik, R. N. Sahoo, S. K. Pradhan, S. Mallick, Indian J. Pharm. Sci. 83 (2021) 794-807 (https://doi.org/10.36468/pharmaceutical-sciences.831)
R. Swain, S. Nandi, R. N. Sahoo, S. S. Swain, S. Mohapatra, S. Mallick. J. Drug Deliv. Sci. Technol. 67 (2021) 102956 (https://doi.org/10.1016/j.jddst.2021.102956)
Z. Jafariazar, N. Jamalinia, F. Ghorbani-Bidkorbeh, S. A. Mortazavi, Iran. J. Pharm. Sci. 14 (2015) 23 (https://doi.org/10.22037/ijpr.2015.1709)
G. Fang, X. Yang, Q. Wang, A. Zhang, B. Tang, Mater. Sci. Eng., C 127 (2021) 112212 (https://doi.org/10.1016/j.msec.2021.112212)
N. Kavanagh, O. I. Corrigan, Int. J. Pharm. 279 (2004) 141 (https://doi.org/10.1016/j.ijpharm.2004.04.016)
B. Vigani, S. Rossi, G. Sandri, M. C. Bonferoni, C. M. Caramella, F. Ferrari, Pharmaceutics 12 (2020) 859 (https://doi.org/10.3390/pharmaceutics1209085)
A. A. El-Bary, H. K. Ibrahim, B. S. Haza’a, I. A. Sharabi, Pharm. Dev. Technol. 24 (2019) 824 (https://doi.org/10.1080/10837450.2019.1602631)
M. Mansour, S. Mansour, N. D. Mortada, S. S. Abd El-Hady, Drug. Dev. Ind. Pharm. 34 (2008):744 (https://doi.org/10.1080/03639040801926030)
M. Tighsazzadeh, J. C. Mitchell, J. S. Boateng, Int. J. Pharm. 566 (2019) 111 (https://doi.org/10.1016/j.ijpharm.2019.05.059).
J. Ye, H. Zhang, H. Wu, C. Wang, X. Shi, J. Xie, J. He, J. Yang, Graefes Arch. Clin. 250 (2012) 1459 (https://doi.org/10.1007/s00417-012-2087-4)
A. Nanda, R. N. Sahoo, A. Pramanik, Colloids Surfaces, B 172 (2018) 555 (https://doi.org/10.1016/j.colsurfb.2018.09.011)
M. R. Abbaspour, B. S. Makhmalzadeh, S. Jalali, Jundishapur J. Nat. Pharm. Prod. 5 (2010) 6 (https://brief.land/jjnpp/articles/72379.html)
P. Talik, J. Piotrowska, U. Hubicka, AAPS Pharm. Sci. Tech. 20 (2019) 187 (https://doi.org/10.1208/s12249-019-1406-z)
R. Mohapatra, S. Senapati, C. Sahoo, S. Mallick, Colloids Surfaces, B 123 (2014)170 (https://doi.org/10.1016/j.colsurfb.2014.09.012)
A. Pramanik, R. N. Sahoo, A. Nanda, Curr. Eye Res. 43 (2018) 828 (https://doi.org/10.17344/acsi.2019.5139)
K. N. Priya, S. Bhattacharyya, P. R. Babu, Dhaka Univ. J. Pharm. Sci. 13 (2014) 75 (https://doi.org/10.3329/dujps.v13i1.21866)
B. Panda, R. Subhadarsini, S. Mallick, Expert Opin. Drug Deliv. 13 (2016) 633 (https://doi.org/10.1517/17425247.2016.1154038)
M. J. Habib, J. A. Rogers, Int. J. Pharm. 44 (1988) 235 (https://doi.org/10.1016/0378-5173(88)90120-2)
J. T. Mitchell-Koch, K. R. Reid, M. E. Meyerhoff, J. Chem. Educ. 85 (2008) 1658 (https://doi.org/10.1021/ed085p1658)
N. A. Farid, G. S. Born, W. V. Kessler, S. M. Shaw, W. E. Lange, Clin. Chem. 21 (1975) 1167 (https://doi.org/10.1093/clinchem/21.8.1167)
W. J. Keller Jr., Am. J. Clin. Pathol. 17 (1947) 415 (https://doi.org/10.1093/ajcp/17.5_ts.415)
W. A. McBryde, J. L. Rohr, J. S. Penciner, J. A. Page, Can. J. Chem. 48 (1970) 2574 (https://doi.org/10.1139/v70-433)
A. Pramanik, R. N. Sahoo, S. Nandi, A. Nanda, S. Mallick, Acta Chim. Slov. 68 (2021) 159-69 (http://dx.doi.org/10.17344/acsi.2020.6298)
P. W. Morrison, C. J. Connon, V. V. Khutoryanskiy, Mol. Pharm. 10 (2013) 756 (https://doi.org/10.1021/mp3005963)
R. N. Sahoo, B. S. Satapathy, S. Mallick, J. Serb. Chem. Soc. 86 (2021) 571 (https://doi.org/10.2298/JSC201209021N)
B. S. Satapathy, A. Patel, R. N. Sahoo, S. Mallick, J. Serb. Chem. Soc. 86 (2021) 51 (https://doi.org/10.2298/JSC200705049S)
P. K. Pawar, D. K. Majumdar, AAPS Pharm. Sci. Tech. 7 (2006) 13 (https://doi.org/10.1208/pt070113)
M. F. Sohail, G. Shahnaz, F. ur Rehman, A. ur Rehman, N. Ullah, U. Amin, G. M. Khan, K. U. Shah, AAPS Pharm. Sci. Tech. 20 (2019) 288 (https://doi.org/10.1208/s12249-019-1484-y)
S. Nandi, A. Ojha, A. Nanda, R. N. Sahoo, R. Swain, K. P. Pattnaik, S. Mallick, Z. Phys. Chem. 236 (2021) 275 (https://doi.org/10.1515/zpch-2021-3081)
E. Larraneta, R. E. Lutton, A. J. Brady, E. M. Vicente‐Pérez, A. D. Woolfson, R. R. Thakur, R.F. Donnelly, Macromol. Mater. Eng. 300 (2015) 586 (https://doi.org/10.1002/mame.201500016)
A. Semalty, M. Semalty, D. Singh, M. S. Rawat, Int. J. Pharm. Sci. Nanotechnol. 3 (2010) 940 (https://doi.org/10.37285/ijpsn.2010.3.2.7)
S. Farias, J. S. Boateng, Int. J. Pharm. 553 (2018) 65 (https://doi.org/10.1016/j.ijpharm.2018.10.025)
R. Mohanty, S. K. Das, N. R. Singh, M. Patri, Zebrafish. 13 (2016) 188 (https://doi.org/10.1089/zeb.2015.1215)
J. A. Castro-Hermida, H. GóMez-Couso, M. E. Ares-Mazás, M. M. Gonzalez-Bedia, N. CastañEda-Cancio, F. J. Otero-Espinar, J. Blanco-Mendez, J. Pharm. Sci. 93 (2004) 197 (https://doi.org/10.1002/jps.10528)
T. Oka, T. Shearer, M. Azuma, Curr. Eye Res. 29 (2004) 27 (https://doi.org/10.1080/02713680490513164)
J. C. Fehrenbacher, M. R. Vasko, D. B. Duarte, Curr. Protoc. Pharmacol. 56 (2012) 541 (https://doi.org/10.1002/0471141755.ph0504s56).