Design of benzimidazoles, benzoxazoles, benzothiazoles and thiazolopyridines as leukotriene A4 hydrolase inhibitors through 3D-QSAR, docking and molecular dynamics Scientific paper
Main Article Content
Abstract
Human leukotriene A4 hydrolase enzyme (LTA4H) catalyses the biotransformation of the inactive precursor leukotriene A4 (LTA4) to the bioactive Leukotriene B4 (LTB4), which causes many inflammatory responses in the human body. Therefore, the selective inhibition of this enzyme becomes a useful strategy for the treatment of several illnesses such as asthma, allergic rhinitis, cardiovascular diseases, and cancer. Herein we report a 3D-QSAR/CoMFA and CoMSIA study on a series of 47 benzimidazoles, benzoxazoles, benzothiazoles and thiazolopyridines reported as potent LTA4H inhibitors. Good statistical parameters were obtained for the best model (q2 = 0.568,
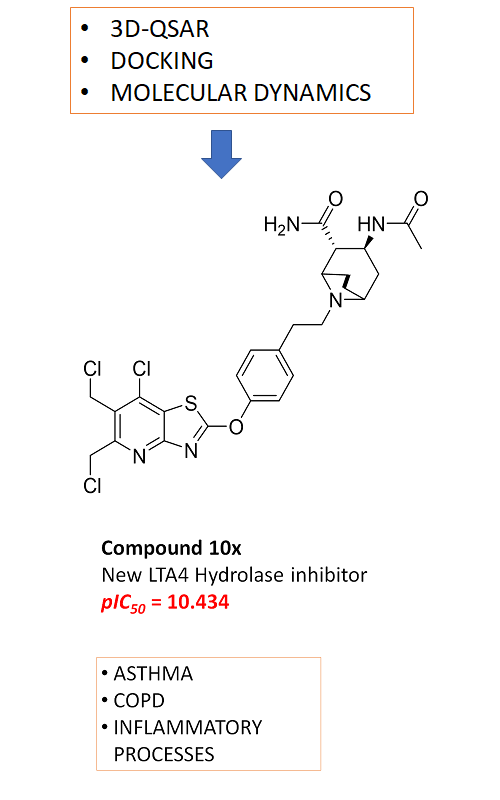
r2ncv = 0.891 and r2test = 0.851). A new series of 10 compounds capable of inhibiting leukotriene A4 hydrolase with high potency was presented. All designed inhibitors showed low IC50 in nano- and sub-nanomolar ranges, when they were evaluated in 3D-QSAR models. Subsequently, the designed molecules, as well as the least and most active compounds were subjected to docking and molecular dynamics studies into LTA4H. In conclusion, we summarised a thorough structure–activity relationship (SAR) of LTA4H inhibitors of heterocyclic structure. These models can be used for the rational proposal of new inhibitors.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Comisión Nacional de Investigación Científica y Tecnológica
Grant numbers 11130701
References
J. Z. Haeggström, A. Rinaldo-Matthis, C. E. Wheelock, A. Wetterholm, Biochem. Biophys. Res. Commun. 396 (2010) 135 (https://doi.org/10.1016/j.bbrc.2010.03.140)
R. J. Snelgrove, Thorax 66 (2011) 550 (https://doi.org/10.1136/thoraxjnl-2011-200234)
N. Gueli, W. Verrusio, A. Linguanti, W. De Santis, N. Canitano, F. Ippoliti, V. Marigliano, M. Cacciafesta, Arch. Gerontol. Geriatr. 52 (2011) e36 (https://doi.org/10.1016/j.archger.2010.04.014)
T. D. Penning, L. J. Askonas, S. W. Djuric, R. A. Haack, S. S. Yu, M. L. Michener, G. G. Krivi, E. Y. Pyla, Bioorg. Med. Chem. Lett. 5 (1995) 2517 (https://doi.org/10.1016/0960-894X(95)00441-U)
N. L. Rao, P. J. Dunford, X. Xue, X. Jiang, K. A. Lundeen, F. Coles, J. P. Riley, K. N. Williams, C. A. Grice, J. P. Edwards, J. Pharmacol. Exp. Ther. 321 (2007) 1154 (https://doi.org/10.1124/jpet.106.115436)
W. Barchuk, J. Lambert, R. Fuhr, J.Z. Jiang, K. Bertelsen, A. Fourie, X. Liu, P.E. Silkoff, E.S. Barnathan, R. Thurmond, Pulm. Pharmacol. Ther. 29 (2014) 15 (https://doi.org/10.1016/j.pupt.2014.06.003)
E. Pontiki, D. Hadjipavlou-Litina, Med. Res. Rev. 28 (2008) 39 (https://doi.org/10.1002/med.20099)
P. R. Bernstein, Am. J. Respir. Crit. Care. Med. 157 (1998) S220 (https://doi.org/10.1164/ajrccm.157.6.mar-3)
L. V. Sonawane, S. B. Bari, Acta Pharm. Sin., B 45 (2010) 615 (http://www.ncbi.nlm.nih.gov/pubmed/20931764)
T. Sundarapandian, J. Shalini, S. Minky, A. Venkatesh, W. L. Keun, Future Med. Chem. 5 (2013) 27 (https://doi.org/10.4155/fmc.12.184)
V. M. Tanis, G. M. Bacani, J. M. Blevitt, C. C. Chrovian, S. Crawford, A. De Leon, A. M. Fourie, L. Gomez, C. A. Grice, K. Herman, Bioorg. Med. Chem. Lett. 22 (2012) 7504 (https://doi.org/10.1016/j.bmcl.2012.10.036)
C. A. Grice, K. L. Tays, B. M. Savall, J. Wei, C. R. Butler, F. U. Axe, S. D. Bembenek, A. M. Fourie, P. J. Dunford, K. Lundeen, J. Med. Chem. 51 (2008) 4150 (https://doi.org/10.1021/jm701575k)
D. R. Davies, B. Mamat, O. T. Magnusson, J. Christensen, M. H. Haraldsson, R. Mishra, B. Pease, E. Hansen, J. Singh, D. Zembower, J. Med. Chem. 52 (2009) 4694 (https://doi.org/10.1021/jm900259h)
M. Lorca, Y. Valdes, H. Chung, J. Romero-Parra, C.D. Pessoa-Mahana, J. Mella, Int. J. Mol. Sci. 20 (2019) 2510 (https://doi.org/10.3390/ijms20102510)
K. Roy, S. Kar, P. Ambure, Chemometr. Intell. Lab. Syst. 145 (2015) 22 (https://doi.org/10.1016/j.chemolab.2015.04.013)
R. Kumari, R. Kumar, A. Lynn, J. Chem. Inf. Model. 54 (2014) 1951 (https://doi.org/10.1021/ci500020m).