Binding of β-casein with fluvastatin and pitavastatin Scientific paper
Main Article Content
Abstract
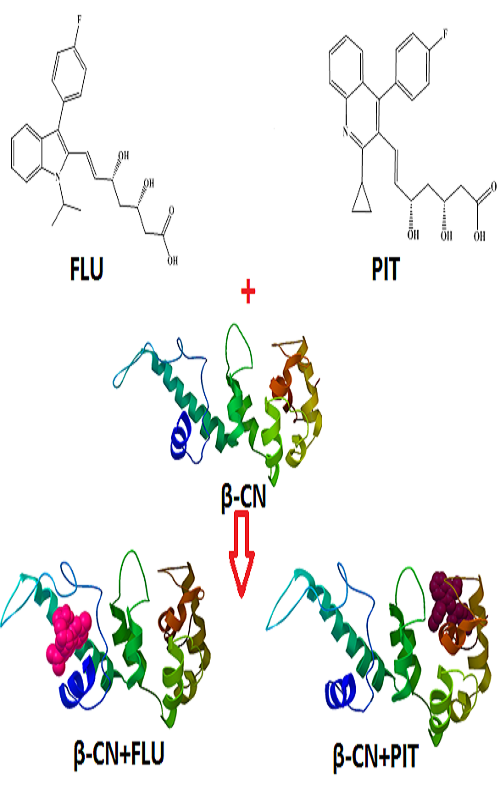
In this work, the binding interaction of fluvastatin (FLU) and pit-avastatin (PIT) with bovine β-casein (β-CN) were performed under physiological conditions (pH 7.2) by fluorescence emission spectroscopy, synchronous fluorescence spectroscopy, Fourier transform infrared spectroscopy (FTIR) and molecular docking methods. Due to the formation of FLU-β-CN and PIT-β-CN complexes, the intrinsic fluorescence of β-CN was quenched. The number of bound FLU and PIT per protein molecule (n) were about 1, also the binding constant of FLU-β-CN and PIT-β-CN complexes were 7.96×104 and 3.44×104 M-1 at 298 K, respectively. This result suggests that the binding affinity of FLU to β-CN was higher than that for PIT. Molecular modelling showed different binding sites for FLU and PIT on β-CN. All these experimental results suggest that β-CN can be used as a carrier protein which delivers FLU and PIT based drugs to target molecules.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
M. Gupta, R. Sharma, A. Kumar, Pharm. Exp. Med. 19 (2019) 259 (https://doi.org/10.1007/s13596-019-00393-x )
J. H. Shi, Q. Wang, D. Q. Pan, T. T. Liu, M. Jiang. J. Biomol. Struct. Dyn. 35 (2017) 1529 (https://doi.org/10.1080/07391102.2016.1188416)
A. L. Toppo, M. Yadav, S. Dhagat, S. Ayothiraman, J. S. Eswari, Ind. J. Biochem. Biophys. 58 (2021) 127
M. S. Khan, Ann. Romanian Soc. Cell Biol. 25 (2021) 6244
J. S. Yu, D. H. Shin, J. Kim, Pharmaceutics 12 (2020) 1133 (https://doi.org/10.3390/pharmaceutics12121133)
Ž. Reiner, M. Hatamipour, M. Banach, M. Pirro, K. Al-Rasadi, Arch. Med. Sci. 16 (2020) 490 (https://dx.doi.org/10.5114%2Faoms.2020.94655)
A. Sahebkar, N. Kiaie, A. M. Gorabi, M. R. Mannarino, V. Bainaconi, T. Jamialahmadi, M. Pirro, M. Banach, Prog. Lipid Res. 84 (2021) 101127 (https://doi.org/10.1016/j.plipres.2021.101127)
S. Rahimi Yazdi, M. Corredig, Food Chem. 132 (2012) 1143 (https://doi.org/10.1016/j.foodchem.2011.11.019)
K. L. Field, B. A. Kimball, J. A. Mennella, G. K. Beauchamp, A. A. Bachmanov, Physiol. Behav. 93 (2008) 189 (https://doi.org/10.1016/j.physbeh.2007.08.010)
Z. Allahdad, M. Varidi, R. Zadmard, A. Akbar, Food Chem. 255 (2018) 187 (https://doi.org/10.1016/j.foodchem.2018.01.143)
H. E. Indyk, B. D. Gill, J. E. Wood, S. Chetikam, T. Kobayashi, J. Food Compos. Anal. 101 (2021) 103946 (https://doi.org/10.1016/j.jfca.2021.103946)
M. Li, R. Kembaren, Y. Ni, J.M. Kleijn, Food Chem. 352 (2021) (https://doi.org/10.1016/j.foodchem.2021.129400)
N. Sarreshtehdari, F.S. Mohseni-Shahri, F. Moeinpour, Luminescence 36 (2021) 360 (https://doi.org/10.1002/bio.3951)
I. Portnaya, U. Cogan, Y. D. Livney, O. Ramon, K. Shimoni, M. Rosenberg, D. Danino, Food Chem. 54 (2006) 5555 (https://doi.org/10.1021/jf060119c)
J. Kaur, L. Katopo, A. Hung, J. Ashton, S. Kasapis, Food Chem. 252 (2018) 163 (https://doi.org/10.1016/j.foodchem.2018.01.091)
D. C. Thorn, S. Meehan, M. Sunde, A. Rekas, S. L. Gras, C. E. MacPhee, C. M. Dobson, M. R. Wilson, J. A. Carver, Biochemistry 44 (2005) 17027 (https://doi.org/10.1021/bi051352r)
L. Condict, J. Kaur, A. Hung, J. Ashton, S. Kasapis, Food Hydrocoll. 89 (2019) 351 (https://doi.org/10.1016/j.foodhyd.2018.10.055)
F. Mehranfar, A. K. Bordbar, H. Parastar, J. Photochem. Photobiol., B 127 (2013) 100 (https://doi.org/10.1016/j.jphotobiol.2013.07.019)
I. Hasni, P. Bourassa, S. Hamdani, G. Samson, R. Carpentier, H. A. Tajmir-Riahi, Food Chem. 126 (2011) 630 (https://doi.org/10.1016/j.foodchem.2010.11.087)
H. Dezhampanah, M. Esmaili, A. Khorshidi, J. Mol. Struct. 1136 (2017) 50 (https://doi.org/10.1016/j.molstruc.2017.01.065)
T. Liao, Y. Zhang, X. Huang, Z. Jiang, X. Tuo, Spectrochim. Acta, A 246 (2021) 119000 (https://doi.org/10.1016/j.saa.2020.119000)
F. Kong, J. Tian, M. Yang, Y. Zheng, X. Cao, X. Yue, Spectrochim. Acta A 243 (2020) (https://doi.org/10.1016/j.saa.2020.118824)
B. Li, R. Fu, H. Tan, Y. Zhang, W. Teng, Z. Li, J. Tian, Spectrochim. Acta, A 259 (2021) 119910 (https://doi.org/10.1016/j.saa.2021.119910)
Q. Wang, C. R. Huang, M. Jiang, Y. Y. Zhu, J. Wang, J. Chen, J. H. Shi, Spectrochim. Acta, A 156 (2016) 155 (https://doi.org/10.1016/j.saa.2015.12.003)
Z. Yin, X. Qie, M. Zeng, Z. Wang, F. Qin, J. Chen, W. Li, Z. He, Food Hydrocoll. 123 (2022) 107177 (https://doi.org/10.1016/j.foodhyd.2021.107177)
F. Azarakhsh, A. Divsalar, A. A. Saboury, A. Eidi, J. Mol. Liq. 333 (2021) 115999 (https://doi.org/10.1016/j.molliq.2021.115999)
G. Ma, C. Tang, X. Sun, J. Zhang, Food Hydrocoll. 113 (2021) 106485 (https://doi.org/10.1016/j.foodhyd.2020.106485)
28. A. Chakraborty, S. Basak, J. Photochem. Photobiol., B 87 (2007) 191 (https://doi.org/10.1016/j.jphotobiol.2007.04.004)
H. Dezhampanah, R. Firouzi, Z. Moradi Shoeili, R. Binazir, J. Mol. Struct. 1205 (2020) 127557 (https://doi.org/10.1016/j.molstruc.2019.127557)
K. Yang, C. Zhou, C. Liao, J. Sun, Y. Wang, R. Guan, J. Neng, P. Sun, LWT 144 (2021) 111225 (https://doi.org/10.1016/j.lwt.2021.111225)
M. Ariyaeifar, H. Amiri Rudbari, M. Sahihi, Z. Kazemi, A. A. Kajani, H. Zali-Boeini, N. Kordestani, G. Bruno, S. Gharaghani, J. Mol. Struct. 1161 (2018) 497 (https://doi.org/10.1016/j.molstruc.2018.02.042)
J. H. Shi, J. Wang, Y. Y. Zhu, J. Chen, J. Lumin. 145 (2014) 643 (https://doi.org/10.1016/j.jlumin.2013.08.042)
J. Hua Shi, D. Qi Pan, X. Xiou Wang, T. T. Liu, M. Jiang, Q. Wang, J. Photochem. Photobiol., B 162 (2016) 14–23 (https://doi.org/10.1016/j.jphotobiol.2016.06.025)
B. Hemmateenejad, M. Shamsipur, F. Samari, T. Khayamian, J. Pharm. Biomed. Anal. 67–68 (2012) 201 (https://doi.org/10.1016/j.jpba.2012.04.012)
H. Bi, L. Tang, X. Gao, J. Jia, H. Lv, J. Lumin. 178 (2016) 72 (https://doi.org/10.1016/j.jlumin.2016.05.048)
S. Gong, C. Yang, J. Zhang, Y. Yu, X. Gu, W. Li, Z. Wang, Food Hydrocoll. 111 (2021) 106223 (https://doi.org/10.1016/j.foodhyd.2020.106223)
P. Bourassa, L. Bekale, H. A. Tajmir-Riahi, J. Biol. Macromol. 70 (2014) 156 (https://doi.org/10.1016/j.ijbiomac.2014.06.038)
S. K. Pawar, S. Jaldappagari, J. Pharm. Anal. 9 (2019) 274 (https://doi.org/10.1016/j.jpha.2019.03.007).