Synthesis of novel menthol derivatives containing 1,2,3-triazole group and their in vitro antibacterial activities Scientific paper
Main Article Content
Abstract
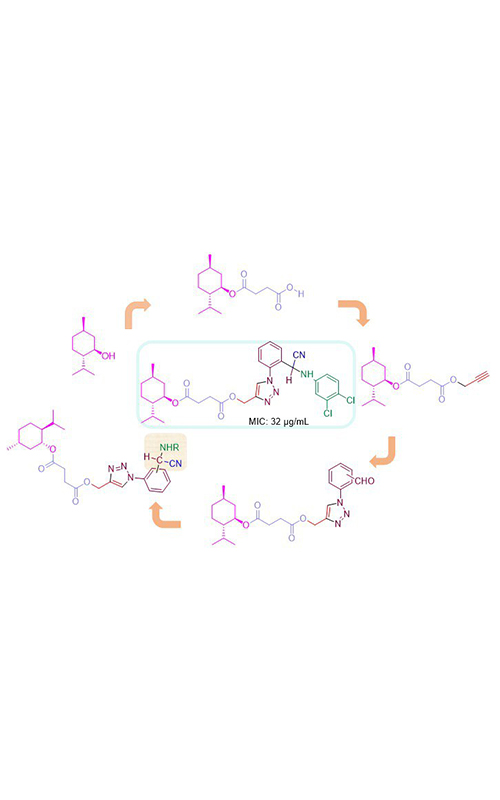
New N-substituted α-aminonitrile derivatives from menthol were synthesized by consecutive succinic ester formation, propargylation, 1,3-dipolar Huisgen cycloaddition and Strecker reaction. The structures of the synthesized compounds were confirmed by diverse spectroscopic techniques including 1H-NMR, 13C-NMR, ESI-MS and IR. The novel synthesized compounds were evaluated for their in vitro antibacterial activities against Staphylococcus aureus as Gram-positive and Escherichia coli as Gram-negative bacteria. These compounds demonstrated a strong inhibitory effect against S. aureus with the minimum inhibitory concentration (MIC) values ranged from 32–128 µg mL-1. Derivatives 6a2, 6b1, 6b4 and 6b5 with a MIC value of 32 µg mL-1 exhibited the best inhibitory effects.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
References
N. Kolassa, Regul. Toxicol. Pharmacol. 65 (2013) 115 (http://dx.doi.org/10.1016/J.YRTPH.2012.11.009)
G. Kamatou, I. Vermaak, A. Viljoen, B. Lawrence, Phytochemistry 96 (2013) 15 (http://dx.doi.org/10.1016/j.phytochem.2013.08.005)
W. A. Russin, J. D. Hoesly, C. E. Elson, M. A. Tanner, M. N. Gould, Carcinogenesis 10 (1989) 2161 (http://dx.doi.org/10.1093/carcin/10.11.2161)
I. Freires, C. Denny, B. Benso, S. Alencar, P. Rosalen, Molecules 20 (2015) 7329 (http://dx.doi.org/10.3390/molecules20047329)
S. Ismailova, E. Mammadbeyli, A. Gurbanov, S. Muradova, Process. Petrochem. Oil Refin. 20 (2019) 168
G. Zengin, Chem. Nat. Compd. 47 (2011) 550 (http://dx.doi.org/10.1007/S10600-011-9994-1)
R. Ejaz, S. Malik, M. Ahmad, H. Ali, S. Choudhry, BCSRJ (2020) 37 (http://dx.doi.org/10.54112/bcsrj.v2020i1.37)
N. S. Vatmurge, B. G. Hazra, V. S. Pore, F. Shirazi, M. V Deshpande, S. Kadreppa, S. Chattopadhyay, R. G. Gonnade, Org. Biomol. Chem. 6 (2008) 3823 (http://dx.doi.org/10.1039/b809221d)
X. Chu, C. Wang, W. Wang, L. Liang, W. Liu, K. Gong, K. Sun, Eur. J. Med. Chem. 166 (2019) 206 (http://dx.doi.org/10.1016/j.ejmech.2019.01.047)
Z. Xu, S. Zhao, Chemistry, Y. Liu, Eur. J. Med. Chem. 183 (2019) 111700 (http://dx.doi.org/10.1016/j.ejmech.2019.111700)
R. Raj, P. Singh, P. Singh, J. Gut, P. Rosenthal, V. Kumar, Eur. J. Med. Chem. 62 (2013) 590 (http://dx.doi.org/10.1016/j.ejmech.2013.01.032)
E. Bonandi, M. S. Christodoulou, G. Fumagalli, D. Perdicchia, G. Rastelli, D. Passarella, Drug Discovery Today 22 (2017) 1572 (http://dx.doi.org/10.1016/j.drudis.2017.05.014)
I. Głowacka, J. Balzarini, A. Wróblewski, Eur. J. Med. Chem. 70 (2013) 703 (http://dx.doi.org/10.1016/j.ejmech.2013.10.057)
J. E. Doiron, C. A. Le, B. K. Ody, J. B. Brace, S. J. Post, N. L. Thacker, H. M. Hill, G. W. Breton, M. J. Mulder, S. Chang, T. M. Bridges, L. Tang, W. Wang, S. M. Rowe, S. G. Aller, M. Turlington, Chem. Eur. J. 25 (2019) 3662 (http://dx.doi.org/10.1002/CHEM.201805919)
R. Reddyrajula, U. Dalimba, Bioorg. Med. Chem. Lett. 30 (2020) 126846 (http://dx.doi.org/10.1016/j.bmcl.2019.126846)
N. Otto, T. Opatz, Chem. Eur. J. 20 (2014) 13064 (http://dx.doi.org/10.1002/CHEM.201403956)
I. Echevarría, M. Vaquero, R. Quesada, G. Espino, Inorg. Chem. Front. 7 (2020) 3092 (http://dx.doi.org/10.1039/D0QI00580K)
K. Babanezhad, P. Salehi, S. Nejad, M. Bararjanian, M. Kaiser, H. Reza, A. Al-Harrasi, Bioorg. Chem. 91 (2019) 103116 (http://dx.doi.org/10.1016/j.bioorg.2019.103116)
J. D. Scott, R. M. Williams, Chem. Rev. 102 (2002) 1669 (http://dx.doi.org/10.1021/CR010212U)
D. Enders, J. Shilvock, Chem. Soc. Rev. 29 (2000) 359 (http://dx.doi.org/10.1039/a908290e)
B. Ganem, Acc. Chem. Res. 42 (2009) 463 (http://dx.doi.org/10.1021/AR800214S)
I. N. Shaikh, K. M. Hosamani, M. M. Kurjogi, Arch. Pharm. (Weinheim) 351 (2018) 1700205 (http://dx.doi.org/10.1002/ardp.201700205)
V. Kouznetsov, C. Galvis, Tetrahedron 74 (2018) 773 (http://dx.doi.org/10.1016/j.tet.2018.01.005)
E. Ezzatzadeh, Z. Hossaini, Nat. Prod. Res. 34 (2018) 923 (http://dx.doi.org/10.1080/14786419.2018.1542389)
R. Dalavai, K. Gomathi, K. Naresh, F. R. Nawaz Khan, Polycycl. Arom. Compd. 42 (2020) 1581 (http://dx.doi.org/10.1080/10406638.2020.1791917)
F. Nemati, P. Salehi, M. Bararjanian, N. Hadian, M. Mohebbi, G. Lauro, D. Ruggiero, S. Terracciano, G. Bifulco, I. Bruno, Bioorg. Med. Chem. Lett. 30 (2020) 127489 (http://dx.doi.org/10.1016/j.bmcl.2020.127489)
P. Khaligh, P. Salehi, M. Bararjanian, A. Aliahmadi, H. Khavasi, S. Nejad-Ebrahimi, Chem. Pharmaceut. Bull. 64 (2016) 1589 (http://dx.doi.org/10.1248/cpb.c16-00463)
J. R. Baker, J. Gilbert, S. Paula, X. Zhu, J. Sakoff, A. Mccluskey, Chem. Eur. 13 (2018) 1447 (http://dx.doi.org/10.1002/cmdc.201800256)
E. Pelkey, G. Gribble, Tetrahedron Lett. 38 (1997) 5603 (http://dx.doi.org/10.1016/S0040-4039(97)01272-0)
N. T. Pokhodylo, V. S. Matiychuk, M. D. Obushak, Synthesis (Stuttgart) (2009) 2321 (http://dx.doi.org/10.1055/S-0029-1216850)
P. A. Procopiou, S. P. D. Baugh, S. S. Flack, G. G. A. Inglis, J. Org. Chem. 63 (1998) 2342 (http://dx.doi.org/10.1021/JO980011Z)
A. Abu-Fayyad, S. Nazzal, Int. J. Pharmaceut. 528 (2017) 463 (http://dx.doi.org/10.1016/j.ijpharm.2017.06.031)
J. Czarnik-Kwaśniak, K. Kwaśniak, K. Tutaj, I. Filiks, Ł. Uram, M. Stompor, S. Wołowiec, J. Drug Deliv. Sci. Technol. 55 (2020) 101424 (http://dx.doi.org/10.1016/J.JDDST.2019.101424)
S. Keskin, M. Balci, Org. Lett. 17 (2015) 964 (http://dx.doi.org/10.1021/ACS.ORGLETT.5B00067)
S. E. Sadat-Ebrahimi, A. Rahmani, M. Mohammadi-Khanaposhtani, N. Jafari, S. Mojtabavi, M. Ali Faramarzi, M. Emadi, A. Yahya-Meymandi, B. Larijani, M. Biglar, M. Mahdavi, Med. Chem. Res. 29 (2020) 868 (http://dx.doi.org/10.1007/s00044-020-02522-7)
J. Wang, X. Liu, X. Feng, Chem. Rev. 111 (2011) 6947 (http://dx.doi.org/10.1021/CR200057T/ASSET/CR200057T.FP.PNG_V03)
H. Y. Guo, Z. A. Chen, Q. K. Shen, Z. S. Quan, J. Enzyme Inhib. Med. Chem. 36 (2021) 1115 (http://dx.doi.org/10.1080/14756366.2021.1890066).