Microwave-assisted synthesis of a series of 4,5-dihydro-1H-pyrazoles endowed with selective COX-1 inhibitory potency Scientific paper
Main Article Content
Abstract
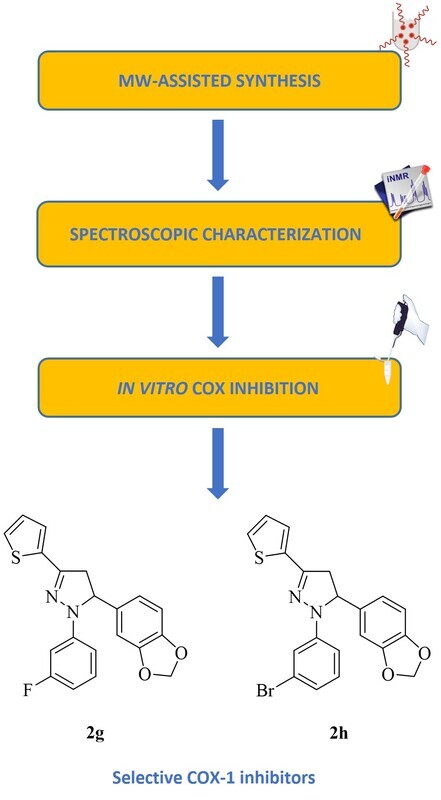
Considerable efforts have been directed towards the discovery of selective cyclooxygenase isoxyme 1 (COX-1) inhibitors due to the recent work highlighting the involvement of COX-1 in the pathogenesis of pain, neuroinflammation, cancer and cardiovascular disorders. In this context, this paper aims to describe 2-pyrazolines endowed with selective COX-1 inhibitory potency. An efficient microwave-assisted synthetic method was applied for the preparation of a series of pyrazolines, which were tested for their COX-1 and cyclooxygenase isoxyme 2 (COX-2) inhibitory effects using a colorimetric assay. The cytotoxic properties of the most potent derivatives on NIH/3T3 fibroblast cells were determined using MTT method. 1-(3-Fluorophenyl)-5-(3,4-methylendioxyphenyl)-3-(2-thienyl)-4,5-dihydro-1H-pyrazole (2g) and 1-(3-bromophenyl)-5-(3,4-methylendioxyphenyl)-3-(2-thienyl)-4,5-dihydro-1H-pyrazole (2h) were determined as selective COX-1 inhibitors. According to the in silico data obtained from Schrödinger’s QikProp module, both compounds are estimated to possess favourable oral bioavailability and drug-likeness. This work could be a rational guideline for further modifications at different sites on 2-pyrazoline motif to bring out a new class of selective COX-1 inhibitors.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Anadolu Üniversitesi
Grant numbers 2207S114
References
T. Schmid, B. Brüne Front. Immunol. 12 (2021) 714042 (https://dx.doi.org/10.3389/fimmu.2021.714042)
L. L. Mazaleuskaya, E. Ricciotti, Adv. Exp. Med. Biol. 1274 (2020) 29 (https://dx.doi.org/10.1007/978-3-030-50621-6_3)
C. S. Williams, M. Mann, R. N. DuBois, Oncogene 18 (1999) 7908 (https://dx.doi.org/10.1038/sj.onc.1203286)
А. Pannunzio, M. Coluccia, Pharmaceuticals 11 (2018) 101 (https://dx.doi.org/10.3390/ph11040101)
V. Sharma, P. Bhatia, O. Alam, M. Javed Naim, F. Nawaz, A. Ahmad Sheikh, M. Jha Bioorg. Chem. 89 (2019) 103007 (https://dx.doi.org/10.1016/j.bioorg.2019.103007)
M. G. Perrone, A. Scilimati, L. Simone, P. Vitale, Curr. Med. Chem. 17 (2010) 3769 (https://dx.doi.org/10.2174/092986710793205408)
E. Caiazzo, A. Ialenti, L. Cicala, C. Vitale, Eur. J. Pharmacol. 848 (2019) 105 (https://dx.doi.org/10.1016/j.ejphar.2019.01.044)
P. Vitale, A. Panella, A. Scilimati, M. G. Perrone, Med. Res. Rev. 36 (2016) 641 (https://dx.doi.org/10.1002/med.21389)
P. Vitale, A. Scilimati, M. G. Perrone, Curr. Med. Chem. 22 (2015) 4271 (https://dx.doi.org/10.2174/0929867322666151029104717)
K. Goto, H. Ochi, Y. Yasunaga, H. Matsuyuki, T. Imayoshi, H. Kusuhara, T. Okumoto, Prostaglandins Other Lipid Mediat. 56 (1998) 245 (https://dx.doi.org/10.1016/s0090-6980(98)00054-9)
J.M. Alex, R. Kumar, J. Enzyme Inhib. Med. Chem. 29 (2014) 427 (https://dx.doi.org/10.3109/14756366.2013.795956)
B. Nehra, S. Rulhania, S. Jaswal, B. Kumar, G. Singh, V. Monga, Eur. J. Med. Chem. 205 (2020) 112666 (https://dx.doi.org/10.1016/j.ejmech.2020.112666)
S. Kumar, S. Bawa, S. Drabu, R. Kumar, H. Gupta, Recent Pat. Anti-Infect. Drug Discov. 4 (2009) 154 (https://dx.doi.org/10.2174/157489109789318569)
M. R. Shaaban, A. S. Mayhoub, A. M. Farag, Expert Opin. Ther. Pat. 22 (2012), 253 (https://dx.doi.org/10.1517/13543776.2012.667403)
А. Marella, R. Ali, T. Alam, R. Saha, O. Tanwar, M. Akhter, M. Shaquiquzzaman, M. M. Mini-Rev. Med. Chem. 13 (2013) 921 (https://dx.doi.org/10.2174/1389557511313060012)
D. Matiadis, M. Sagnou, Int. J. Mol. Sci. 21 (2020) 5507 (https://dx.doi.org/10.3390/ijms21155507)
C. Cusan, G. Spalluto, M. Prato, M. Adams, A. Bodensieck, R. Bauer, A. Tubaro, P. Bernardi, T. Da Ros, Farmaco 60 (2005) 327 (https://dx.doi.org/10.1016/J.FARMAC.2004.09.002)
M. V. R. Reddy, V. K. Billa, V. R. Pallela, M. R. Mallireddigari, R. Boominathan, J.L. Gabriel, E. P. Reddy, Bioorg. Med. Chem. 16 (2008) 3907 (https://dx.doi.org/10.1016/j.bmc.2008.01.047)
R. Fioravanti, A. Bolasco, F. Manna, F. Rossi, F. Orallo, F. Ortuso, S. Alcaro, R. Cirilli, Eur. J. Med. Chem. 45 (2010) 6135 (https://dx.doi.org/10.1016/j.ejmech.2010.10.005)
S. Carradori, D. Secci, A. Bolasco, C. De Monte, M. Yáñez, Arch. Pharm. Chem. Life Sci. 345 (2012) 973 (https://dx.doi.org/10.1002/ardp.201200249)
M. A. El-Sayed, N. I. Abdel-Aziz, A. A. Abdel-Aziz, A. S. El-Azab, K. E. ElTahir, Bioorg. Med. Chem. 20 (2012) 3306 (https://dx.doi.org/10.1016/j.bmc.2012.03.044)
M. Yu, H. Yang, K. Wu, Y. Ji, L. Ju, X. Lu, Bioorg. Med. Chem. 22 (2014) 4109 (https://dx.doi.org/10.1016/j.bmc.2014.05.059)
K. R. A. Abdellatif, M. A. Abdelgawad, M. B. Labib, T. H. Zidan, Bioorg. Med. Chem. Lett. 25 (2015) 5787 (https://dx.doi.org/10.1016/j.bmcl.2015.10.047)
K. R. A. Abdellatif, H. A. H. Elshemy, A. A. Azoz, Bioorg. Chem. 63 (2015) 13 (https://dx.doi.org/10.1016/j.bioorg.2015.09.002)
M. A. Abdel-Sayed, S. M. Bayomi, M. A. El-Sherbeny, N. I. Abdel-Aziz, K. E. ElTahir, G. S. Shehatou, A. A. Abdel-Aziz, Bioorg. Med. Chem. 24 (2016) 2032 (https://dx.doi.org/10.1016/j.bmc.2016.03.032)
K. R. A. Abdellatif, M. T. Elsaady, S. A. Abdel-Aziz, A. H. Abusabaa, J. Enzyme Inhib. Med. Chem. 31 (2016) 1545 (https://dx.doi.org/10.3109/14756366.2016.1158168)
M. Lutz, J. Clin. Pharmacol. 59 (2019) 1433 (https://dx.doi.org/10.1002/jcph.1512)
А. Özdemir, B. Sever, M. D. Altıntop, E. Kaya Tilki, M. Dikmen, Molecules 23 (2018) 2151 (https://dx.doi.org/10.3390/molecules23092151)
А. Özdemir, M. D. Altıntop, Z. A. Kaplancıklı, G. Turan-Zitouni, G. Akalın Çiftçi, Ş. Ulusoylar Yıldırım, J. Enzyme Inhib. Med. Chem. 28 (2013) 1221 (https://dx.doi.org/10.3109/14756366.2012.724682)
T. Mosmann, J. Immunol. Methods 16 (1983) 55 (https://dx.doi.org/10.1016/0022-1759(83)90303-4)
E. Berrino, C. T. Supuran, Expert Opin. Drug Discov. 13 (2018) 861 (https://dx.doi.org/10.1080/17460441.2018.1494721)
T. L. Lambat, P. K. P. G. Chopra, S. H. Mahmood, Curr. Org. Chem. 24 (2020) 2527 (https://dx.doi.org/10.2174/1385272824999200622114919)
M. Henary, C. Kananda, L. Rotolo, B. Savino, E. A. Owens, G. Cravotto, RSC Adv. 10 (2020) 14170 (https://dx.doi.org/10.1039/D0RA01378A)
M. B. Gawande, S. N. Shelke, R. Zboril, R. S. Varma, Acc. Chem. Res. 47 (2014) 1338 (https://dx.doi.org/10.1021/ar400309b)
J. M. Kremsner, A. Stadler, A Chemist’s Guide to Microwave Synthesis, 3rd ed., Anton Paar GmbH, Graz, 2018, p. 300
Y. Wang, J. Xing, Y. Xu, N. Zhou, J. Peng, Z. Xiong, X. Liu, X. Luo, C. Luo, K. Chen, M. Zheng, H. Jiang, Q. Rev. Biophys. 48 (2015) 488 (https://dx.doi.org/10.1017/S0033583515000190)
Schrödinger Release 2022-2, Schrödinger, LLC, New York (https://www.schrodinger.com/)
C. Lohmann, S. Hüwel, H. J. Galla, J. Drug Target. 10 (2002) 263 (https://dx.doi.org/10.1080/10611860290031903)
T. J. Hou, X. J. Xu, J. Chem. Inf. Comput. Sci. 43 (2003) 2137 (https://dx.doi.org/10.1021/ci034134i)
S. Shahbazi, T. R. Sahrawat, M. Ray, S. Dash, D. Kar, S. Singh, PLoS ONE 11 (2016) e0156156 (https://dx.doi.org/10.1371/journal.pone.0156156)
F. Neumaier, B. D. Zlatopolskiy, B. Neumaier, Pharmaceutics 13 (2021) 1542 (https://dx.doi.org/10.3390/pharmaceutics13101542).