Antidiabetic potential of simple carbamate derivatives: Comparative experimental and computational study Scientific paper
Main Article Content
Abstract
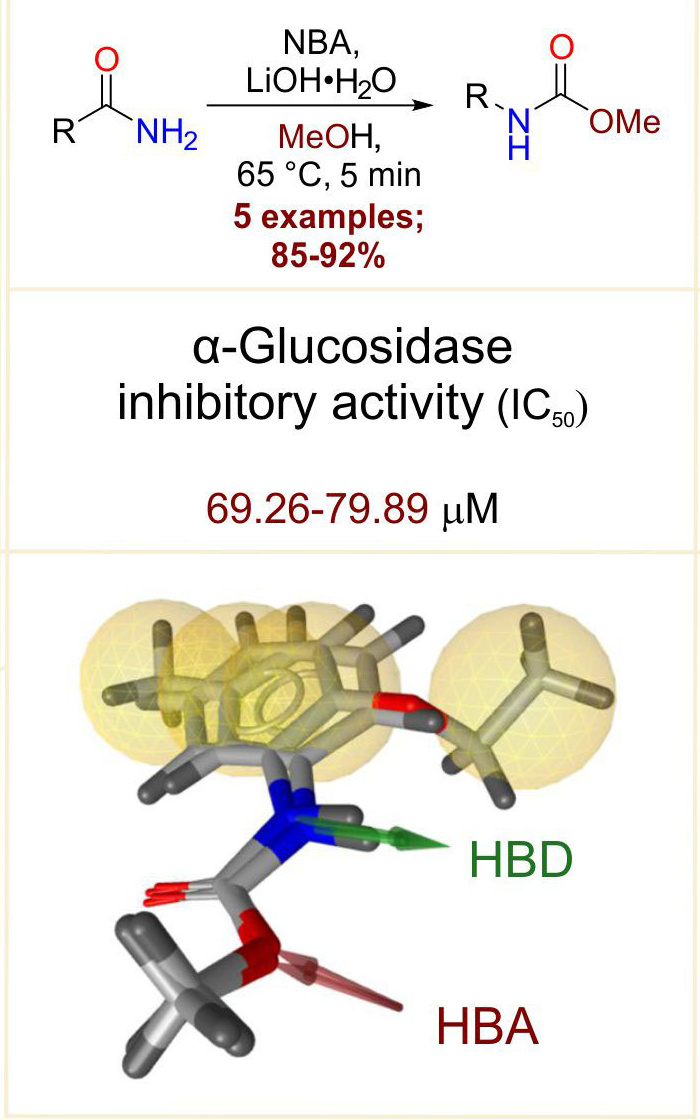
With the increasing global burden of diabetes mellitus type 2, the search for the new drugs, with better pharmacological profile is continued. As a part of this surge, the synthesis, pharmacological in vitro and computational evaluation of five, simple carbamate derivatives, against carbohydrate digestive enzyme α-glucosidase, is disclosed herein. Results of the experimental and computational assessment indicated that examined carbamates deterred the activity of α-glucosidase with acceptable IC50 values ranging from 65.34 to 79.89 µM compared to a standard drug acarbose (109.71 µM). Similarly, the studied compounds displayed in silico binding affinity for α-glucosidase enzyme with significant binding energies. Preliminary toxicity profiles of studied carbamates against three cancerous cell lines indicated their poor activity, suggesting that significant structural modifications have to be made to improve their anticancer efficiency. Results of the present study indicate that the examined carbamates were able to virtually or experimentally interact with an important target of diabetes mellitus type 2. Additionally, a new pharmacophore model is proposed featuring hydrogen bond donating carbamate –NH group, hydrogen bond accepting carbamate –OCH3 group and hydrophobic stabilization of aromatic moieties.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-68/2022-14/200026 -
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-68/2022-14/200043
References
X. Lin, Y. Xu, X. Pan, J. Xu, Y. Ding, X. Sun, X. Song, Y. Ren, P.-F. Shan, Sci. Rep. 10 (2020) 14790 (https://doi.org/10.1038/s41598-020-71908-9)
H. Rasouli, R. Yarani, F. Pociot, J. P. Popović-Djordjević, Pharmacol. Res. 155 (2020) 104723 (https://doi.org/10.1016/j.phrs.2020.104723)
J. Størling, F. Pociot, Genes 8 (2017) 72 (https://doi.org/10.3390/genes8020072)
R. V. Cohen, J. C. Pinheiro, C. A. Achiavon. J. E. Salles, B. L. Wajchenberg, D. E. Cummings, Diabetes Care 35 (2012) 1420 (https://doi.org/10.2337/dc11-2289)
N. S. Artzi, S. Shilo, E. Hadar, H. Rossman, S. Barbash-Hazan, A. Ben-Haroush, R. D. Balicer, B. Feldman, A. Wiznitzer, E. Segal, Nat. Med. 26 (2020) 71 (https://doi.org/10.1038/s41591-019-0724-8)
J. B. Popovic-Djordjevic, I. I. Jevtić, T. P. Stanojkovic, Curr. Med. Chem. 25 (2018) 2140 (https://doi.org/10.2174/0929867325666171205145309)
H. Rasouli, R. Khodarahmi, S. Mohammad, B. Hosseini Ghazvini, H. Adibi, Food Funct. 8 (2017) 1942 (https://doi.org/10.1039/C7FO00220C)
J. B. Popović-Djordjević, I. I. Jevtić, N. Dj. Grozdanić, S. B. Šegan, M. V. Zlatović, M. D. Ivanović, T. P. Stanojković, J. Enz. Inhib. Med. Chem. 32 (2017) 298 (https://doi.org/10.1080/14756366.2016.1250754)
J. N. Gorantla, S. Maniganda, S. Pengthaisong, L. Ngiwsara, P. Sawangareetrakul, S. Chokchaisiri, P. Kittakoop, J. Svasti, J. R. Ketudat Cairns, ACS Omega 6 (2021) 25710 (https://doi.org/10.1021/acsomega.1c03928)
N. Kausar, S. Ullah, M. Aqeel Khan, H. Zafar, A.-t.-Wahab, M. I. Choudhary, S. Yousuf, Bioorg. Chem. 106 (2021) 104499 (https://doi.org/10.1016/j.bioorg.2020.104499)
A. K. Ghosh, M. Brindisi, J. Med. Chem. 58 (2015) 2895 (https://doi.org/10.1021/jm501371s)
M. Chandrasekhar, G. S. Prasad, C. Venkataramaiah, C. Naga Raju, K. Seshaiah, W. Rajendra, Mol. Divers. 23 (2019) 723 (https://doi.org/10.1007/s11030-018-9906-4)
J. Ma, N. Lu, W. Quin, R. Xu, Y. Wang, X. Chen, Ecotoxicol. Environ. Saf. 63 (2006) 268 (https://doi.org/10.1016/j.ecoenv.2004.12.002)
M. D. Stephens, N. Yodsanit, C. Melander, Org. Biomol. Chem. 14 (2016) 6853 (https://doi.org/10.1039/C6OB00706F)
S. Clarke, F. Mulcahy, HIV Medicine 1 (2000) 15 (https://doi.org/10.1046/j.1468-1293.2000.00004.x)
C. Fortin, V. Joly, Expert Rev. Anti Infec. Ther. 2 (2004) 671 (https://doi.org/10.1586/14789072.2.5.671)
N. Y. Rakhmanina, J. N. Van den Anker, Expert Opin. Drug Metab. Toxicol. 6 (2010) 95 (https://doi.org/10.1517/17425250903483207)
N. Pathak, K. Fatima, S. Singh, D. Mishra, A. C. Gupta, Y. Kumar, D. Chanda, D. U. Bawankule, K. Shanker, F. Khan, A. Gupta, S. Luqman, A. S. Negi, J. Steroid Bioch. Mol. Biol. 194 (2019) 105457 (https://doi.org/10.1016/j.jsbmb.2019.105457)
U. Košak, N. Strašek, D. Knez, M. Jukič, S. Žakelj, A. Zahirović, A. Pišlar, X. Brazzlotto, F. Nachon, J. Kos, S. Gobec, Eur. J. Med. Chem. 197(2020) 112282 (https://doi.org/10.1016/j.ejmech.2020.112282)
M. Saeedi, M. Raeeisi-Nafchi, S. Sobhani, S. S. Mirfazli, M. Zardkanlou, S. Mojtabavi, M. A. Faramarzi, T. Akbarzadeh, Mol. Divers. 25 (2021) 2399 (https://doi.org/10.1007/s11030-020-10137-8)
J. D. Durrant, J. A. McCammon, BMC Biol. 9 (2011) 1 (https://doi.org/10.1186/1741-7007-9-71)
J. E. Kerrigan, in In Silico Models for Drug Discovery. Methods in Molecular Biology (Methods and Protocols), S. Kortagere, Ed., Humana Press, Totowa, NJ, 2013, p. 95 (https://doi.org/10.1007/978-1-62703-342-8_7)
I. I. Jevtić, Lj. Došen-Mićović, E. R. Ivanović, M. D. Ivanović, Synthesis 48 (2016) 1550 (https://doi.org/10.1055/s-0036-1588985)
P. Mccue, Y. I. Kwon, K. Shetty, J. Food Biochem. 29 (2005) 278 (https://doi.org/10.1111/j.1745-4514.2005.00020.x)
V. Marković, N. Debeljak, T. Stanojković, B. Kolundžija, D. Sladić, M. Vujčić, B. Janović, N. Tanić, M. Perović, V. Tešić, J. Antić, M. D. Joksović. Eur. J. Med. Chem. 89 (2015) 401 (https://doi.org/10.1016/j.ejmech.2014.10.055)
V. Roig-Zamboni, B. Cobucci-Ponzano, R. Iacono, M. Carmina Ferrara, S. Germany, Y. Bourne, G. Parenti, M. Moracci, G. Sulzenbacher, Nat. Commun. 8 (2017) 1 (https://dx.doi.org/10.1038%2Fs41467-017-01263-3)
H. R. Mohammadi-Motlagh, Y. Shokohina, M. Majarrab, H. Rasouli, A. Mostafaie, Biomed. Pharmacother. 93 (2017) 117 (https://doi.org/10.1016/j.biopha.2017.06.013)
H. Rasouli, M. Mehrabi, S. S. Arab, R. Khodarahmi, J. Iran. Chem. Soc. 14 (2017) 2023 (https://doi.org/10.1007/s13738-017-1140-y)
I. I. Jevtić, K. Savić Vujović, D. Srebro, S. Vučković, M. D. Ivanović, S. V. Kostić-Ra-jačić, Pharmacol. Rep. 72 (2020) 1069 (https://doi.org/10.1007/s43440-020-00121-2)
V. Janganati, N. Reddy Penthala, N. Reddy Madadi, Z. Chen, P.A. Crooks, Bioorg. Med. Chem. Lett. 24 (2014) 3499 (https://doi.org/10.1016/j.bmcl.2014.05.059)
I. Kufareva, R. Abagyan, Methods of Protein Structure Comparison in Homology Modeling. Methods in Molecular Biology (Methods and Protocols), A. Orry, R. Abagyan, Eds., Humana Press, Totowa, NJ, 2011, p. 231 (https://doi.org/10.1007/978-1-61779-588-6_10)
Y. Xie, J. An, G. Yang, G. Wu, Y. Zhang, L. Cui, Y. Feng, J. Biol. Chem. 289 (2014) 7994 (https://doi.org/10.1074/jbc.M113.536045).