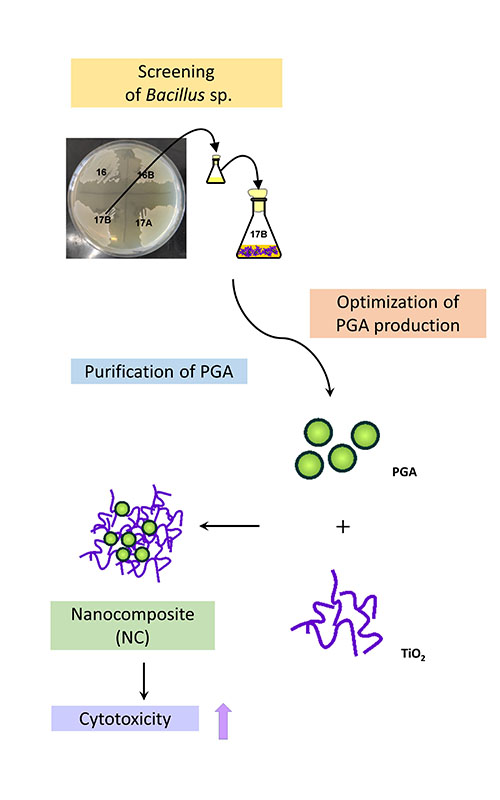
A novel PGA/TiO2 nanocomposite prepared with poly(γ-glutamic acid) from the newly isolated Bacillus subtilis 17B strain
Main Article Content
Abstract
Poly(γ-glutamic acid) (PGA), naturally produced by Bacillus species, is a biodegradable, non-toxic, biocompatible and non-immunogenic negatively charged polymer. Due to its properties, it has found various applications in the food, cosmetic and pharmaceutical industries. In this work, Bacillus subtilis 17B was selected as the best PGA producer among fifty wild-types Bacillus strains tested and characterized as a glutamate-independent producer. The production of PGA by the newly identified strain was optimized and increased tenfold using the Box–Behnken experimental design. The purity of PGA after recovery and purification from the fermentation broth was confirmed by SDS-PAGE followed by methylene blue staining. PGA was characterized by ESI MS and used for the preparation of a new nanocomposite with TiO2. The synthesis of PGA/TiO2 nanocomposite, its structural analysis, and cytotoxic effect on the cervical cancer cell line (HeLa cell) was investigated to determine the potential anti-cancer usage of this newly prepared material. It is encouraging that PGA/TiO2 nanocomposite showed an increased cytotoxic effect compared to TiO2 alone.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-68/2022-14/200026;451-03-68/2022-14/200168;450-03-68/2022-14/200017
References
I. Bajaj, R. Singhal, Bioresour. Technol. 102 (2011) 5551 (https://doi.org/10.1016/j.biortech.2011.02.047)
W. E. Hanby, H. N. Rydon, Biochem. J. 40 (1946) 297 (https://doi.org/10.1042/bj0400297)
S. bin Park, M. H. Sung, H. Uyama, D. K. Han, Prog. Polym. Sci. 113 (2021) 101341 (https://doi.org/10.1016/j.progpolymsci.2020.101341)
C. Wang, M. Feng, J. Deng, Y. Zhao, X. Zeng, L. Han, S. Pan, C. Wu, Int. J. Pharm. 398 (2010) 237 (https://doi.org/10.1016/j.ijpharm.2010.07.048)
M. Moriyama, H. Uyama, A. J. van der Vlies, U. Hasegawa, Colloid Polym. Sci. 293 (2015) 1245 (https://doi.org/10.1007/s00396-015-3516-9)
D. Ziental, B. Czarczynska-Goslinska, D. T. Mlynarczyk, A. Glowacka-Sobotta, B. Stanisz, T. Goslinski, L. Sobotta, Nanomaterials 10 (2020) 387 (https://doi.org/10.3390/nano10020387)
F. F. Hezayen, B. H. A. Rehm, B. J. Tindall, A. Steinbüchel, Int. J. Syst. Evol. Microbiol. 51 (2001) 1133 (https://doi.org/10.1099/00207713-51-3-1133)
T. Candela, M. Moya, M. Haustant, A. Fouet, Can. J. Microbiol. 55 (2009) 627 (https://doi.org/10.1139/w09-003)
J. H. Do, H. N. Chang, S. Y. Lee, Biotechnol. Bioeng. 76 (2001) 219 (https://doi.org/10.1002/bit.1186)
M. Schallmey, A. Singh, O. P. Ward, Can. J. Microbiol. 17 (2004) 1 (https://doi.org/10.1139/W03-076)
A. Goto, M. Kunioka, Biosci. Biotechnol. Biochem. 56 (1992) 37 (https://doi.org/10.1271/bbb.56.1031)
G. Du, G. Yang, Y. Qu, J. Chen, S. Lun, Process Biochem. 40 (2005) 2143 (https://doi.org/10.1016/j.procbio.2004.08.005)
F. Shi, Z. Xu, P. Cen, Biotechnol. Bioprocess Eng. 11 (2006) 251 (https://doi.org/10.1007/BF02932039)
C. Zhang, D. Wu, X. Qiu, Sci. Rep. 8 (2018) 17934 (https://doi.org/10.1038/s41598-018-36439-4)
Y. H. Ko, R. A. Gross, Biotechnol. Bioeng. 57 (1998) 430 (https://doi.org/10.1002/(SICI)1097-0290(19980220)57:4<430::AID-BIT6>3.0.CO;2-N)
D. A. Hopwood, M. J. Bibb, K. F. Chater, T. Kieser, C. J. Bruton, H. M. Kieser, D. J. Lydiate, C. P. Smith, M. Ward, H. Schrempf, Genetic Manipulation of Streptomyces: A Laboratory Manual, John Innes Foundation, Foulsham, 1985
D. J. Lane, 16S/23S rRNA Sequencing. in Nucleic acid techniques in bacterial systematics, E. Stackebrandt and M. Goodfellow, Eds., Wiley, New York, 1991
S. F. Altschul, T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, D. J. Lipman, Nucleic Acids Res. 25 (1997) 3389 (https://doi.org/10.1093/nar/25.17.3389)
C. Park, J.-C. Choi, Y.-H. Choi, H. Nakamura, K. Shimanouchi, T. Horiuchi, H. Misono, T. Sewaki, K. Soda, M. Ashiuchi, M.-H. Sung, J. Mol. Catal., B 35 (2005) 128 (https://doi.org/10.1016/j.molcatb.2005.06.007)
U. K. Laemmli, Nature 227 (1970) 680 (https://doi.org/10.1038/227680a0)
F. Yamaguchi, Y. Ogawa, M. Kikuchi, K. Yuasa, H. Motai, Biosci. Biotechnol. Biochem. 60 (1996) 255 (https://doi.org/10.1271/bbb.60.255)
T. Rajh, A. E. Ostafin, O. I. Micic, D. M. Tiede, M. C. Thurnauer, J. Phys. Chem. 100 (1996) 4538 (https://doi.org/10.1021/jp952002p)
R. C. Thompson, Inorg. Chem. 23 (1984) 1794 (https://doi.org/10.1021/ic00181a003)
D. Lin, S. D. Story, S. L. Walker, Q. Huang, W. Liang, P. Cai, Environ. Pollut. 228 (2017) 35 (https://doi.org/10.1016/j.envpol.2017.05.025)
P. Skehan, R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J. T. Warren, H. Bokesch, S. Kenney, M. R. Boyd, J. Natl. Cancer Inst. 82 (1990) 1107 (https://doi.org/10.1093/jnci/82.13.1107)
G. A. Birrer, A.-M. Cromwick, R. A. Gross, Int. J. Biol. Macromol. 16 (1994) 265 (https://doi.org/10.1016/0141-8130(94)90032-9)
J. M. Buescher, A. Margaritis, Crit. Rev. Biotechnol. 27 (2007) 1 (https://doi.org/10.1080/07388550601166458)
H. Xu, M. Jiang, H. Li, D. Lu, P. Ouyang, Process Biochem. 40 (2005) 519 (https://doi.org/10.1016/j.procbio.2003.09.025)
D. Wang, J.-S. Hwang, D.-H. Kim, S. Lee, D.-H. Kim, M.-H. Joe, Process Biochem. 92 (2020) 164 (https://doi.org/10.1016/j.procbio.2019.11.034)
W. Zeng, G. Chen, Y. Guo, B. Zhang, M. Dong, Y. Wu, J. Wang, Z. Che, Z. Liang, AMB Express 7 (2017) 213 (https://doi.org/10.1186/s13568-017-0512-0)
M. Cao, W. Geng, L. Liu, C. Song, H. Xie, W. Guo, Y. Jin, S. Wang, Bioresour. Technol. 102 (2011) 4251 (https://doi.org/10.1016/j.biortech.2010.12.065)
M. Ashiuchi, K. Soda, H. Misono, Biochem. Biophys. Res. Commun. 263 (1999) 6 (https://doi.org/10.1006/bbrc.1999.1298)
F. Yamaguchi, Y. Ogawa, M. Kikuchi, K. Yuasa, H. Motai, Biosci. Biotechnol. Biochem. 60 (1996) 255 (https://doi.org/10.1271/bbb.60.255)
C. Ho, C. Lam, M. Chan, R. Cheung, L. Law, L. Lit, K. Ng, M. Suen, H. Tai, Clin. Biochem. Rev. 24 (2003) (https://pubmed.ncbi.nlm.nih.gov/18568044)
I. Kwiecień, I. Radecka, M. Kowalczuk, K. Jelonek, A. Orchel, G. Adamus, J. Am. Soc. Mass Spectrom. 28 (2017) 2223 (https://doi.org/10.1007/s13361-017-1731-y)
G. Kedia, D. Hill, R. Hill, I. Radecka, J. Nanosci. Nanotechnol. 10 (2010) 5926 (https://doi.org/10.1166/jnn.2010.2614)
A. R. Bhat, V. U. Irorere, T. Bartlett, D. Hill, G. Kedia, M. R. Morris, D. Charalampopoulos, I. Radecka, AMB Express 3 (2013) 1 (https://doi.org/10.1186/2191-0855-3-36)
S. W. Bruun, A. Kohler, I. Adt, G. D. Sockalingum, M. Manfait, H. Martens, Appl. Spectrosc. 60 (2006) 1029 (https://doi.org/10.1366/000370206778397371)
A. S. Adeleye, A. A. Keller, Environ. Sci. Technol. 50 (2016) 12258 (https://doi.org/10.1021/acs.est.6b03684)
G. R. Wiese T. W. Healy, J. Colloid Interface Sci. 52 (1975) 452 (https://doi.org/10.1016/0021-9797(75)90270-2)
M. Hamzeh, G. I. Sunahara, Toxicology in Vitro 27 (2013) 864 (https://doi.org/10.1016/j.tiv.2012.12.018)
S. Jafari, B. Mahyad, H. Hashemzadeh, S. Janfaza, T. Gholikhani, L. Tayebi, Int. J. Nanomedicine 15 (2020) 3447 (https://doi.org/10.2147/IJN.S249441).