Flavonoid derivatives as anticancer moiety and its effect on cancer cell lines: An updated review Survey
Main Article Content
Abstract
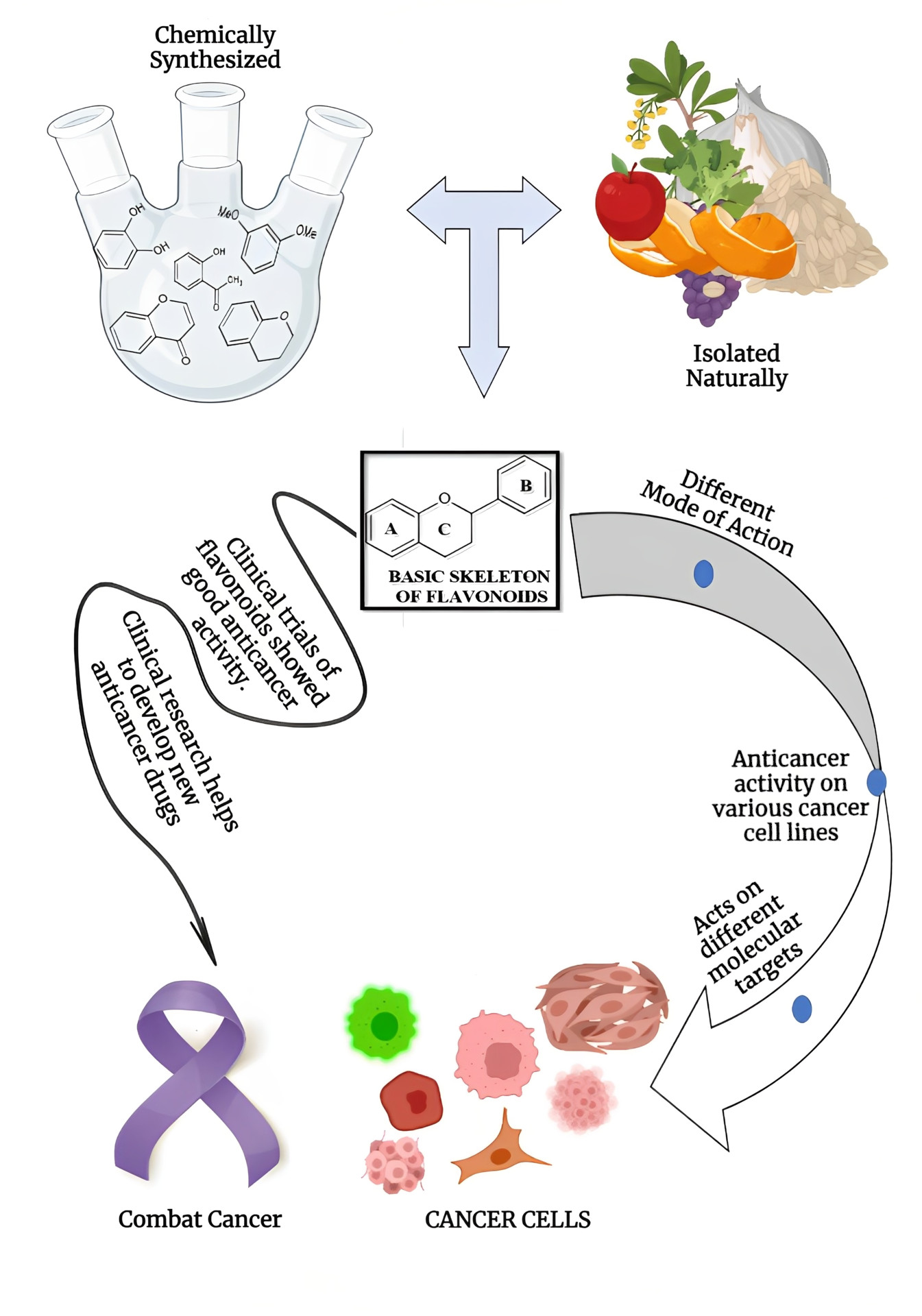
Cancer is now considered the number one leading cause of premature death in industrialized countries. Chemotherapy drugs are quite expensive and cause multiple side effects. Natural products have been studied in depth for their potential as anticancer agents because of their remarkable chemical variability. Among the various natural metabolites, flavonoids are secondary metabolites that are extensively present in nature, have potent anti-cancer properties, have few adverse effects, and also show synergistic benefits. Numerous laboratories are diligently investigating the chemistry and biology of novel flavonoid derivatives due to the demand for and value of these drugs. In this survay, we have summarized clinical trials of various flavonoids, molecular pathways against various cancer cell lines and recent updates on the anticancer activity of flavonoid derivatives against various cancer cells synthesized by various methods, more studies are needed to develop the following mentioned flavonoid derivatives as an anticancer drug.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
Y. Ouyang, J. Li, X. Chen, X. Fu, S. Sun, Q. Wu, Biomolecules 11 (2021) 894 (http://dx.doi.org/10.3390/biom11060894)
S. S. Qi, J. H. Sun, H. H. Yu, S. Q. Yu, Drug Deliv 24 (2017) 1909–1926 (http://dx.doi.org/10.1080/10717544.2017.1410256)
M. Lorscheider, A. Gaudin, J. Nakhle, K. L. Veiman, J. Richard, C. Chassaing, Ther Deliv 12 (2021) 55–76 (http://dx.doi.org/10.4155/tde-2020-0079)
S. Hussain, A. Singh, S. U. Nazir, S. Tulsyan, A. Khan, R. Kumar, N. Bashir, P. Tanwar, R. Mehrotra, J Cell Biochem 120 (2019) 14213–14225 (http://dx.doi.org/10.1002/jcb.28782)
Z. Zhang, J. Shi, E. C. Nice, C. Huang, Z. Shi, Antioxidants 10 (2021) 1138 (http://dx.doi.org/10.3390/antiox10071138)
A. N. Panche, A. D. Diwan, S. R. Chandra, J Nutr Sci 5 (2016) e47 (http://dx.doi.org/10.1017/jns.2016.41)
S. Kumar, A. K. Pandey, The Scientific World Journal 2013 (2013) 1–16 (http://dx.doi.org/10.1155/2013/162750)
Z. F. Fan, S. T. Ho, R. Wen, Y. Fu, L. Zhang, J. Wang, C. Hu, P. C. Shaw, Y. Liu, M. S. Cheng, Molecules 24 (2019) (http://dx.doi.org/10.3390/molecules24173180)
R. K. Singla, A. K. Dubey, A. Garg, R. K. Sharma, M. Fiorino, S. M. Ameen, M. A. Haddad, M. Al-Hiary, JAOAC Int. 102 (2019) 1397–1400 (http://dx.doi.org/10.5740/jaoacint.19-0133)
D. Veeramuthu, W. R. T. Raja, N. A. Al-Dhabi, I. Savarimuthu, Flavonoids: Anticancer Properties, in Flavonoids - From Biosynthesis to Human Health, InTech, 2017 (http://dx.doi.org/10.5772/68095)
A. Rauf, M. Imran, I. A. Khan, M. ur-Rehman, S. A. Gilani, Z. Mehmood, M. S. Mubarak, Phytotherapy Research 32 (2018) 2109–2130 (http://dx.doi.org/10.1002/ptr.6155)
A. U. Khan, H. S. Dagur, M. Khan, N. Malik, M. Alam, M. Mushtaque, European Journal of Medicinal Chemistry Reports 3 (2021) 100010 (http://dx.doi.org/10.1016/j.ejmcr.2021.100010)
D. M. Kopustinskiene, V. Jakstas, A. Savickas, J. Bernatoniene, Nutrients 12 (2020) 457 (http://dx.doi.org/10.3390/nu12020457)
A. Liskova, M. Samec, L. Koklesova, A. Brockmueller, K. Zhai, B. Abdellatif, M. Siddiqui, K. Biringer, E. Kudela, M. Pec, L. K. Gadanec, M. Šudomová, S. T. S. Hassan, A. Zulli, M. Shakibaei, F. A. Giordano, D. Büsselberg, O. Golubnitschaja & P. Kubatka, EPMA Journal 12 (2021) 155–176 (http://dx.doi.org/10.1007/s13167-021-00242-5)
M. K. Chahar, N. Sharma, M. P. Dobhal, Y. C. Joshi, Pharmacog. Rev. 5 (2011) 1–12 (http://dx.doi.org/10.4103/0973-7847.79093)
D. Kashyap, S. Mittal, K. Sak, P. Singhal, H. S. Tuli, Tumor Biology 37 (2016) 12927–12939 (http://dx.doi.org/10.1007/s13277-016-5184-x)
F. Wang, L. Wang, C. Qu, L. Chen, Y. Geng, C. Cheng, S. Yu, D. Wang, L. Yang, Z. Meng, Z. Chen, BMC Cancer 21 (2021) 396 (http://dx.doi.org/10.1186/s12885-021-08158-z)
Z. Javed, K. Khan, J. Herrera-Bravo, S. Naeem, M. J. Iqbal, Q. Raza, H. Sadia, S. Raza, M. Bhinder, D. Calina, J. Sharifi-Rad, W. C. Cho, Cancer Cell Int. 22 (2022) 239 (http://dx.doi.org/10.1186/s12935-022-02663-2)
Z. Nouri, S. Fakhri, K. Nouri, C. E. Wallace, M. H. Farzaei, A. Bishayee, Cancers (Basel) 12 (2020) 2276 (http://dx.doi.org/10.3390/cancers12082276)
P. Pandey, F. Khan, H. A. Qari, M. Oves, Pharmaceuticals 14 (2021) 1069(http://dx.doi.org/10.3390/ph14111069)
M. J. Tuorkey, European Journal of Cancer Prevention 25 (2016) 65–76 (http://dx.doi.org/10.1097/CEJ.0000000000000128)
S. Hu, L. Huang, L. Meng, H. Sun, W. Zhang, Y. Xu, Mol. Med. Rep. 12 (2015) 6745–6751 (http://dx.doi.org/10.3892/mmr.2015.4269)
J. L. Wang, Q. Quan, R. Ji, X. Y. Guo, J. M. Zhang, X. Li, Y. G. Liu, Biomedicine and Pharmacotherapy 108 (2018) 925–933 (http://dx.doi.org/10.1016/j.biopha.2018.09.105)
H. W. Zhang, J. J. Hu, R. Q. Fu, X. Liu, Y. H. Zhang, J. Li, L. Liu, Y. N. Li, Q. Deng, Q. S. Luo, Q. Ouyang, N. Gao, Sci. Rep. 8 (2018) 11255 (http://dx.doi.org/10.1038/s41598-018-29308-7)
S. Rampogu, R. G. Gajula, K. W. Lee, Biomedicine and Pharmacotherapy 141 (2021) 111808 (http://dx.doi.org/10.1016/j.biopha.2021.111808)
J. Sharifi-Rad, J. Herrera-Bravo, L. A. Salazar, S. Shaheen, S. Abdulmajid Ayatollahi, F. Kobarfard, M. Imran, A. Imran, L. Custódio, M. Dolores López, M. Schoebitz, M. Martorell, M. Kumar, H. Ansar Rasul Suleria, W. C. Cho, Evidence-Based Complementary and Alternative Medicine 2021 (2021) 9935451 (http://dx.doi.org/10.1155/2021/9935451)
X. Hou, H. Du, X. Quan, L. Shi, Q. Zhang, Y. Wu, Y. Liu, J. Xiao, Y. Li, L. Lu, X. Ai, M. Zhan, S. Yuan, L. Sun, Front. Pharmacol. 9 (2018) (http://dx.doi.org/10.3389/fphar.2018.00021)
X. Yan, M. Qi, P. Li, Y. Zhan, H. Shao, Cell. Biosci. 7 (2017) 50 (http://dx.doi.org/10.1186/s13578-017-0179-x)
R. A. Fikroh, S. Matsjeh, C. Anwar, Molekul 15 (2020) 34–39 (http://dx.doi.org/10.20884/1.jm.2020.15.1.558)
J. I. Zwicker, B. L. Schlechter, J. D. Stopa, H. A. Liebman, A. Aggarwal, M. Puligandla, T. Caughey, K. A. Bauer, N. Kuemmerle, E. Wong, T. Wun, M. McLaughlin, M. Hidalgo, D. Neuberg, B. Furie, R. Flaumenhaft, JCI Insight 4 (2019) (http://dx.doi.org/10.1172/jci.insight.125851)
US National Library of Medicine, ClinicalTrials.gov, Purple Grape Juice in Improving Vascular Health in Childhood Cancer Survivors (https://clinicaltrials.gov/study/NCT01043939) Accessed October 27, 2022. https://clinicaltrials.gov/ct2/show/NCT01043939
US National Library of Medicine, ClinicalTrials.gov, Prostate Cancer Prevention Trial With Quercetin and Genistein (QUERGEN) (https://clinicaltrials.gov/study/NCT01538316) Accessed October 27, 2022.
US National Library of Medicine, ClinicalTrials.gov, Effect of Quercetin in Prevention and Treatment of Oral Mucositis (https://clinicaltrials.gov/study/NCT01732393) Accessed October 27, 2022.
US National Library of Medicine, ClinicalTrials.gov, Clinical Evaluation of Furocyst in Patients With Poly Cystic Ovary Syndrome (https://clinicaltrials.gov/study/NCT02789488) Accessed October 27, 2022.
US National Library of Medicine, ClinicalTrials.gov, Green Tea Anticancer Mechanisms in Smokers (https://clinicaltrials.gov/study//NCT01162642) Accessed October 27, 2022.
H. D. Sesso, J. E. Manson, A. K. Aragaki, P. M. Rist, L. G. Johnson, G. Friedenberg, T. Copeland, A. Clar, S. Mora, M. V. Moorthy, A. Sarkissian, W. R. Carrick, G. L. Anderson, J. E. Manson, et al., American Journal of Clinical Nutrition 115 (2022) 1490–1500 (http://dx.doi.org/10.1093/AJCN/NQAC055)
B. Ngameni, K. Cedric, A. T. Mbaveng, M. Erdoğan, I. Simo, V. Kuete, A. Daştan, Bioorg. Med. Chem. Lett. 35 (2021) 127827 (http://dx.doi.org/10.1016/j.bmcl.2021.127827)
A. Pangal, Y. Mujahid, B. Desai, J. A. Shaikh, K. Ahmed, Current Chemistry Letters 11 (2022) 105–112 (http://dx.doi.org/10.5267/j.ccl.2021.8.004)
S. Mirzaei, F. Hadizadeh, F. Eisvand, F. Mosaffa, R. Ghodsi, J. Mol. Struct. 1202 (2020) 127310 (http://dx.doi.org/10.1016/j.molstruc.2019.127310)
Q. Wang, X. Hu, W. Shi, H. Long, H. Wang, Bioorg. Med. Chem. Lett. 69 (2022) 128799 (http://dx.doi.org/10.1016/j.bmcl.2022.128799)
S. Mayer, P. Keglevich, P. Ábrányi-Balogh, Á. Szigetvári, M. Dékány, C. Szántay, L. Hazai, Molecules 25 (2020) 888 (http://dx.doi.org/10.3390/molecules25040888)
Parvinder Kaur, Ajmer Singh Grewal, Deepti Pandita, Deepti Pandita, Biointerface Res. Appl. Chem. 13 (2022) 150 (http://dx.doi.org/10.33263/BRIAC132.150)
S. Rahimzadeh Oskuei, S. Mirzaei, M. Reza Jafari-Nik, F. Hadizadeh, F. Eisvand, F. Mosaffa, R. Ghodsi, Bioorg. Chem. 112 (2021) 104904 (http://dx.doi.org/10.1016/j.bioorg.2021.104904)
A. P. Sarkate, V. S. Dofe, S. v. Tiwari, D. K. Lokwani, K. S. Karnik, D. D. Kamble, M. H. S. H. Ansari, S. Dodamani, S. S. Jalalpure, J. N. Sangshetti, R. Azad, P. V. L. S. Burra, S. v. Bhandari, Bioorg. Med. Chem. Lett. 40 (2021) 127916 (http://dx.doi.org/10.1016/j.bmcl.2021.127916)
X. Yan, J. Song, M. Yu, H. L. Sun, H. Hao, Bioorg. Chem. 96 (2020) 103613(http://dx.doi.org/10.1016/j.bioorg.2020.103613)
N. M. Thorat, A. P. Sarkate, D. K. Lokwani, S. v. Tiwari, R. Azad, S. R. Thopate, Mol. Divers. 25 (2021) 937–948 (http://dx.doi.org/10.1007/s11030-020-10079-1)
R. Liu, X. Deng, Y. Peng, W. Feng, R. Xiong, Y. Zou, X. Lei, X. Zheng, Z. Xie, G. Tang, Bioorg. Chem. 96 (2020) 103652 (http://dx.doi.org/10.1016/j.bioorg.2020.103652)
J. Kozłowska, B. Potaniec, D. Baczyńska, B. Zarowska, M. Anioł, Molecules 24 (2019) 4129 (http://dx.doi.org/10.3390/molecules24224129)
E. Assirey, A. Alsaggaf, A. Naqvi, Z. Moussa, R. M. Okasha, T. H. Afifi, A. S. Abd-El-Aziz, Molecules 25 (2020) 544 (http://dx.doi.org/10.3390/molecules25030544)
B. A. Al-Oudat, M. A. Alqudah, S. A. Audat, Q. A. Al-Balas, T. El-Elimat, M. A. Hassan, I. N. Frhat, M. M. Azaizeh, Drug Des. Devel. Ther. 13 (2019) 423–433 (http://dx.doi.org/10.2147/DDDT.S189476)
Y. Hou, W. Kuang, W. Min, Z. Liu, F. Zhang, K. Yuan, X. Wang, C. Sun, H. Cheng, L. Wang, Y. Xiao, S. Pu, G. Z. Xin, P. Yang, J. Med. Chem. 64 (2021) 14942–14954 (http://dx.doi.org/10.1021/acs.jmedchem.1c00087)
V. K. Kumar, V. swamy Puli, K. R. S. Prasad, G. Sridhar, Chemical Data Collections 33 (2021) 100696 (http://dx.doi.org/10.1016/j.cdc.2021.100696)
S. V. S. da Silva, O. M. Barboza, J. T. Souza, É. N. Soares, C. C. dos Santos, L. V. Pacheco, I. P. Santos, T. B. D. S. Magalhães, M. B. P. Soares, E. T. Guimarães, C. S. Meira, S. L. Costa, V. D. A. da Silva, L. L. B. de Santana, A. de F. Santos Júnior, Molecules 26 (2021) 6923 (http://dx.doi.org/10.3390/molecules26226923)
G. Zhong, J. Shen, Z. Chen, Z. Lin, L. Long, J. Wu, C. Long, S. Huang, P. Lian, G. Luo, Molecules 27 (2022) 879 (http://dx.doi.org/10.3390/molecules27030879)
D. Insuasty, S. García, R. Abonia, B. Insuasty, J. Quiroga, M. Nogueras, J. Cobo, G. L. Borosky, K. K. Laali, Arch. Pharm. (Weinheim) 354 (2021) 2100094 (http://dx.doi.org/10.1002/ardp.202100094)
C. F. Lu, S. H. Wang, X. J. Pang, T. Zhu, H. L. Li, Q. R. Li, Q. Y. Li, Y. F. Gu, Z. Y. Mu, M. J. Jin, Y. R. Li, Y. Y. Hu, Y. B. Zhang, J. Song, S. Y. Zhang, Molecules 25 (2020) 5530 (http://dx.doi.org/10.3390/molecules25235530).