Preparation and in-vitro evaluation of single and bi-layered beeswax-based microparticles for colon-specific delivery of mesalamine Scientific paper
Main Article Content
Abstract
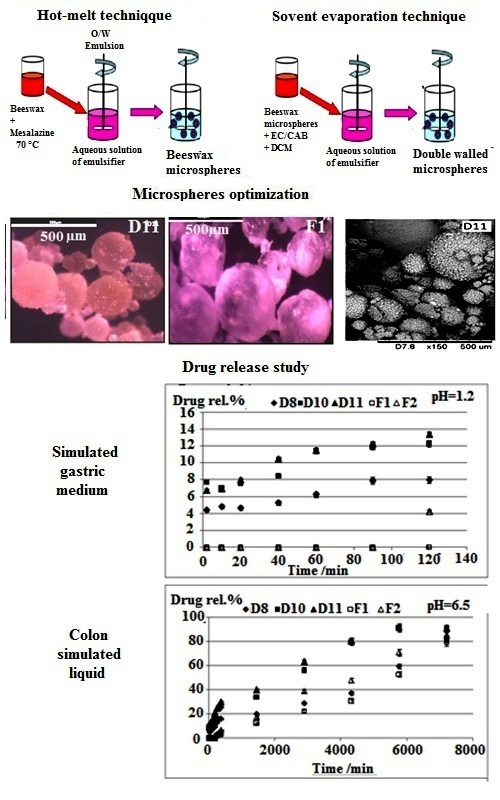
Beeswax is selected as a natural coating material for the development of new colon specific drug delivery systems charged by mesalamine. In a first step, beeswax microparticles are prepared using hot-melt process of microencapsulation where drug:beeswax ratio, stirring speed, emulsifier concentration and pH of external phase are varied for the optimization of the drug entrapment and microparticles’ morphology. The effect of the nature of the emulsifier is also discussed by studying the hydrophilic–lipophilic balance (HLB) value. In a second step, to obtain delayed delivery systems, bi-layered microspheres are elaborated by the process of emulsion–solvent evaporation using ethylcellulose or cellulose acetate butyrate as outer enteric coating layer. All formulations are characterized by infrared spectroscopy, X-ray diffraction, scanning electron microscopy and optical microscopy. The drug release is established in simulated gastric, small bowel and colon liquids and the release mechanism is discussed by applying the Korsmeyer–Peppas model.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
References
A.K. Dash, Mesalamine, in Analytical Profiles of Drug Substances and Excipients, Brittain Harry G, Ed., Academic Press, Cambridge, MA, 1998, pp. 209–242 (https://www.elsevier.com/books/analytical-profiles-of-drug-substances-and-excipients/brittain/978-0-12-260825-4)
M. Ham, A. C. Moss, Expert Rev. Clin. Pharmacol. 5 (2012) 113 (https://doi.org/10.1586/ecp.12.2)
A. H. Teruel, I. G. Alvarez, M. Bermejo, V. Merino, M. D. Marcos, F. Sancenon, M. Alvarez, R. M. Mañez, Int. J. Mol. Sci. 21 (2020) 6502 (https://doi.org/10.3390/ijms21186502)
S. Hua, Front. Pharmacol. 11 (2020) 524 (https://doi.org/10.3389/fphar.2020.00524)
A. K. Philip, B.Philip, Oman Med. J. 25 (2010) 79 (https://doi.org/10.5001/omj.2010.24)
R. Katta, D. Raja, S. Bharath, B. B.V. Raj, Int. J. App. Pharm. 9 (2017) 1 (https://doi.org/10.22159/ijap.2017v9i4.17326)
I. R. Wilding, S. S. Davis, M. Bakhshaee, H. N. Stevens, R. A. Sparrow, J. Brennan, J. Pharm. Res. 9 (1992) 654 (https://doi.org/10.1023/a:1015806211556)
B. S. Souza, H. R. Marcelino, F. Alexandrino, S. C. C. Urtiga, K. C. H. Silva, D. C. F. Soares, E. S. T. Egito, Appl. Sci. 9 (2019) 3519 (https://doi.org/10.3390/app9173519)
A. El Kaoutari, F. Armougom, D. Raoult, B. Henrissat, Med. Sci. (Paris) 30 (2014) 259 (https://doi.org/10.1051/medsci/20143003013)
S. L. Kosaraju, Crit. Rev. Food. Sci. Nutr. 45 (2005) 251 (https://doi.org/10.1080/10408690490478091)
Y. Karrout, C. Neut, D. Wils, F. Siepmann, L. Deremaux, M.-P. Flament, L. Dubreuil, P. Desreumaux, J. Siepmann, Eur. J. Pharm. Sci. 37 (2009) 427 (https://doi.org/10.1016/j.ejps.2009.03.014)
L. Jin, Y-C. Ding, Y. Zhang, X-Q. Xu, Q. Cao, Drug Des. Devel. Ther. 10 (2016) 2021 (https://doi.org/10.2147/DDDT.S107283)
A. P. Tulloch, Bee World 61 (1980) 47 (https://doi.org/10.1080/0005772x.1980.11097776)
F. Fratini, G. Cilia, A. Turchi, A. Felicioli, Asian Pac. J. Trop. Med. 9 (2016) 839 (https://doi.org/10.1016/j.apjtm.2016.07.003)
M. Ranjha, H. Khan, S. Naseem, J. Mater. Sci.: Mater. Med. 21 (2010) 1621 (https://doi.org/10.1007/s10856-010-4034-4)
D.V. Gowda, M. Manjunatha, V. Balmurlidhara, S. Mohammed Khan, Pharm. Lettre 2 (2010) 232 (https://www.scholarsresearchlibrary.com/articles/study-on-encapsulation-of-ranolazine-in-bees-wax-microspherespreparation-characterization-and-release-kinetics-ofmicrosp.pdf)
V. Subha, W. Arulsha, S. Kirubanandan, S. Renganathan. MOJ Drug. Des. Develop. Ther. 2 (2018) 156 (https://doi.org/10.15406/mojddt.2018.02.00042)
N. A. Elechi, H. C. Mital, Int. J. Pharm. Sci. Res. 3 (2012) 1632 (http://dx.doi.org/10.13040/IJPSR.0975-8232.3(6).1632-36)
C. O. Nnadi, A. A. Attama, L. O. Ugwu, Int. J. Res. Med. Health Sci. 1 (2013) 31 (https://www.ijsk.org/uploads/3/1/1/7/3117743/3_design_and_evaluation_of_sustained_release_potential_of_diclofenac_potassium_contained_in_beeswax_matrix.pdf)
A. G. Press, I. A. Hauptmann, L. Hauptmann, B. Fuchs, M. Fuchs, K. Ewe, G. Ramadori, Aliment. Pharmacol. Ther. 12 (1998) 673. (http://dx.doi.org/10.1046/j.1365-2036.1998.00358.x)
S. Nugent, D. Kumar, D. Rampton, D. Evans, Gut 48 (2001) 571 (http://dx.doi.org/10.1136/gut.48.4.571)
A. Newton, N. Kumar, Interdiscip. J. Microinflammation 1 (2014) 1 (https://www.omicsonline.org/open-access-pdfs/ibd-impact-of-colonic-ph-onset-of-action-and-other-factors-in-modern-therapeutic-approach-ijm.1000116.pdf)
J. Fallingborg, P. Pedersen, B.A. Jacobsen, Dig. Dis. Sci. 43 (1998) 702 (http://dx.doi.org/10.1023/a:1018893409596)
A. Gokhale, Pharm. Technol. Eur. 26 (2014) 38 (https://www.researchgate.net/publication/285996771_Achieving_zero-order_release_kinetics_using_multi-step_diffusion-based_drug_delivery)
S. Dash, P. N. Murthy, L. Nath, P. Chowdhury, Acta Pol. Pharm. 67 (2010) 217 (https://www.ptfarm.pl/pub/File/Acta_Poloniae/2010/3/217.pdf)
M. Paarakh, P. Ani Jose, C. M. Setty, G. V. Peter, I. J. P. R. T. 8 (2018) 12 (https://www.ijprt.org/index.php/pub/article/view/85/82)
R. Gouda, H. Baishya, Q. Zhao, J. Develop. Drugs 6 (2017) 1 (http://dx.doi.org/10.4172/2329-6631.1000171)
J. O. Hinze, AIChE J. 1 (1955) 289 (http://dx.doi.org/10.1002/AIC.690010303)
D. L. French, J.W. Mauger Pharm. Res. 10 (1993) 1285 (https://doi.org/10.1023/A:1018909527659)
R. Dinarvand, S. H. Moghadam, A. Sheikhi, F. Atyabi, J. Microencapsul. 22 (2005) 139 (https://doi.org/10.1080/02652040400026392)
A. Müllertz, A. Ogbonna, S. Ren, T. Rades, J. Pharm. Pharmacol. 62 (2010) 1622 (http://dx.doi.org/10.1111/j.2042-7158.2010.01107.x.)
A. Hassan Al-Hmoud, E. Ibrahim Nasser, E. I. El-Hallous, Afr. J. Pharm. Pharmacol. 8 (2014) 364 (https://academicjournals.org/journal/AJPP/article-full-text-pdf/725A2B243946)
R. B. Pedada, E. Vanka, A.M.S. Sudhakar Babu, PK. Desu, PR. Bharathi, PV. Rao, PharmaTutor 1 (2013) 60 (https://www.pharmatutor.org/pdf_download/pdf/1(2)-enhancement-of-solubility-an-over-view.pdf)
A. K. Hassan J. Pharm. Sci. 80 (2018) 334 (http://dx.doi.org/10.4172/pharmaceutical-sciences.1000362)
The HLB System: A Time-Saving Guide To Emulsifier Selection, rev. ed., ICI Americas,Wilmington, DE, 1984, pp.5–6 (http://www.scientificspectator.com/documents/personal%20care%20spectator/The%20HLB%20Book%20ICI.pdf)
E. Kim, W. G. Cho, J. Korean Appl. Sci. Technol. 31 (2014) 203 (http://dx.doi.org/10.12925/jkocs.2014.31.2.203)
S. A. Nour, N. S. Abdelmalak, M. J. Naguib, Drug Deliv. 22 (2015) 286 (https://doi.org/10.3109/10717544.2014.889779)
W. M. Obeidat, J. C. Price, J. Microencapsul. 20 (2003) 57 (https://doi.org/10.1080/0265204021000022716)
D. Kaushik, K. Sharma, S. Sardana, Indian J. Pharm. Educ. Res. 50 (2016) S106 (https://www.ijper.org/article/420)
W. Banabid, F. Djerboua, A. Maiza, Z. ElBahri, M. Baitiche, Indian J. Pharm. Educ. 51 (2017) S46 (http://dx.doi.org/10.5530/ijper.51.2s.49)
A. Bucio, R. Moreno-Tovar, L. Bucio, J. Espinosa-Dávila, F. Anguebes-Franceschi, Coatings 11 (2021) 261 (https://doi.org/10.3390/coatings11030261)
N. M. Mahajan, DR. D. M. Sakarkar, A. S. Manmode, Int. J. Pharm. Pharmacol. Sci. 3 (2011) 208 (https://innovareacademics.in/journal/ijpps/Vol3Issue4/2581.pdf)
A M. Hillery, A.W. Lloyd, J. Swarbrick, Drug Delivery and Targeting for Pharmacists and Pharmaceutical Scientists, ISBN 0-203-34655-6 (Adobe eReader Format), Taylor & Francis e-Library, 2005 (https://doi.org/10.1201/b12801)
A. Gazzaniga, P. Iamartino, G. Maffione, M. E. Sangalli, Int. J. Pharmaceutics 108 (1994) 77 (https://doi.org/10.1016/0378-5173(94)90418-9)
M. K. Chourasia, S. K. Jain, J. Pharm. Pharmaceut. Sci. 6 (2003) 33 (https://sites.ualberta.ca/~csps/JPPS6(1)/S.Chourasia/colon.pdf)
P. L. Ritger, N. A. Peppas, J. Controlled Rel. 5 (1987) 23 (https://doi.org/10.1016/0168-3659(87)90034-4)
J. Siepmann, N A. Peppas, Int. J. Pharm. 418 (2011) 6 (https://doi.org/10.1016/j.ijpharm.2011.03.051)
M. Thakare, B. Israel, S. T. Garner, H. Ahmed, P. Garner, D. Elder, J. C. Price, and A. C. Capomacchia, Pharm. Dev. Technol. 18 (2013) 1213 (https://doi.org/10.3109/10837450.2011.620969)
A. G. Prasanth, A. S. Kumar, B. S. Shruthi, S. Subramanian, Mater. Res. Express 6 (2019) 125427 (https://doi.org/10.1088/2053-1591/ab5811)
M. Padmaa Paarakh, P. A. Jose, C. M. Setty, G. V. Peter Christopher, Int. J. Pharm. Res. Technol. (IJPRT) 8 (2018) 12 (https://ijprt.org/index.php/pub/article/view/85/82).