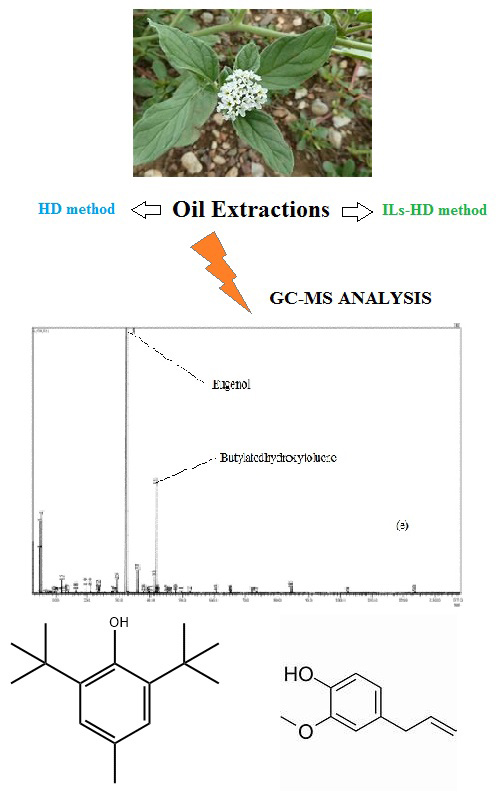
Co-detection of eugenol and butylated hydroxyltoluene by green and selective hydrodistillation of Heliotropium europaeum L. using ionic liquids as additives Scientific paper
Main Article Content
Abstract
This study is the beginning of the research that focuses on unconventional ionic liquids (ILs) hydrodistillation (HD) extraction of the essential oil of Heliotropium europaeum L. using ILs as additives. Two ILs based on 1-butyl-3-methylimidazolium were used by switching the anions (Cl- and PF6-). The effect of mass percent of the added ILs on its yield and composition was evaluated. Compared to the conventional HD, ILs-HD gives a higher yield of essential oils (0.10–0.36 %). Particularly, with [C4mim][PF6], the observation of morphological changes using scanning electron microscopy (SEM) confirmed the effectiveness of the ionic liquid in this distillation process. The GC–MS analysis of essential oils (EOs) revealed the presence of sixty-six compounds in HD, ILs-HD methods. Gas chromatography–mass spectrometry analysis of the EOs revealed the predominance of eugenol (1.70–72.35 %), butylated hydroxytoluene (8.95–65.39 %) and phytol (18.20 %). The new distillation methods of H. europaeum with ILs identifies more compounds (50 compounds in ILs-HD [C4mim][PF6]; 22 compounds in ILs-HD ([C4mim][Cl]) than conventional hydrodistillation (25 compounds in HD). Therefore, the ILs-based hydrodistillation approach is superior in improving the production of EOs. It is important to emphasize that the data presented in this study are not yet available for any of this Algerian Heliotropium species of genus and present the great potential of this medicinal plant as a source of novel bioactive extracts with possible therapeutic uses.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
J. Sharifi-Rad, A. Sureda, G. C. Tenore, M. Daglia, M. Sharifi-Rad, M. Valussi, R. Tundis, M. Sharifi-Rad, M. R. Loizzo, A. O. Ademiluyi, Molecules 22 (2017) 70 (https://doi.org/10.3390/molecules22010070)
A. M. Abd El-Gawad, A. G. El Gendy, A. I. Elshamy, E. A. Omer, J. Essent. Oil Bear. Plants 19 (2016) 1684 (https://doi.org/10.1080/0972060X.2016.1205523)
A. I. Elshamy, A. M. Abd‐ElGawad, Y. A. El‐Amier, A. E. N. G. El Gendy, S. L. Al‐Rowaily, Flavour Fragr. J. 34 (2019) 316 (https://doi.org/10.1002/ffj.3512)
G. Lei, P. Mao, M. He, L. Wang, X. Liu, A. Zhang, J. Chem. Technol. Biotechnol. 91 (2016) 1896 (https://doi.org/10.1002/jctb.4785)
G. Lei, L. Wang, X. Liu, A. Zhang, J. Chem. Eng. Data 61 (2016) 2499 (https://doi.org/10.1021/acs.jced.6b00205)
K. B. Śmigielski, M. Majewska, A. Kunicka‐Styczyńska, M. Szczęsna‐Antczak, R. Gruska, Ł. Stańczyk, J. Food Qual. 34 (2014) 219 (https://doi.org/10.1111/jfq.12092)
J. Jiao, Q. Y. Gai, Y. J. Fu, Y. G. Zu, M. Luo, C. J. Zhao, C. Y. Li,. Separ.Purific. Technol. 107 (2013) 228 (https://doi.org/10.1016/j.seppur.2013.01.009)
C. Chiappe, B. Melai, G. Flamini, L. Pistelli, , RSC Adv. 5 (2015) 69894 (https://doi.org/10.1039/C5RA12649E)
L. Wang, M. Bai, Y. Qin, B. Liu, Y. Wang, Y. Zhou, Molecules 23 (2018) 2309 (https://doi.org/10.3390/molecules23092309)
Kew, Plants of the World 2023 (accessed on 22/06/2023), https://powo.science.kew.org/results?q=Heliotropium europaeum
F. Selvi, M. Bigazzi, Flora 196 (2001) 269 (https://doi.org/10.1016/S0367-2530(17)30056-7)
P. Quézel, S. Santa, Nouvelle flore de l’Algérie et des Régions Désertiques Méridionales, Vol. 2, Centre National de la Recherche Scientifique, Paris, 1963 (https://www.ipni.org/p/20008139-1)
R. Qureshi, G.R. Bhatti, Fitoterapia 79 (2008) 468 (https://doi.org/10.1016/j.fitote.2008.03.010)
C. Wiart Medicinal plants of Asia and the Pacific, CRC Press, Boca Raton, FL, 2006 (https://doi.org/10.1201/9781420006803)
M. Thulin, Flora of Somalia. Pteridophyta; Gymnospamae; Angiospamae (Annonacae-Fabaceae), Vol. 1, CBC Publishing press, Harare, 1993 (ISBN: 9780947643553)
G. Asprey, P. Thornton, The West Ind. Med. J. 4 (1955) 69 (https://caymannature.files.wordpress.com/2019/08/medicinal-plants-jamaica-1953_asprey-thornton.pdf)
G. H. Schmelzer, A. Gurib-Fakim, Plant Resources of Tropical Africa. Medicinal Plants 1, PROTA Foundation, Wageningen, 2008 (ISBN: 9789057822049).
M. Saeedi, K. Morteza-Semnani, Chem. Nat. Comp. 45 (2009) 98 (https://doi.org/10.1007/s10600-009-9239-8)
M. Reina, A. Gonzalez‑Coloma, C. Gutirrez, R. Cabrera, J. Henriquez, L. Villarroel, Phytochemistry 46 (1997) 845 (https://doi.org/10.1016/S0031-9422(97)00354-3)
E. Abdel‑Sattar, F. M. Harraz, S. M. Al‑Ansari, S. El‑Mekkawy, C. Ichino, H. Kiyohara. J. Nat. Med. 63 (2009) 232 (https://doi.org/10.1007/s11418-008-0305-5)
H. Khan, M.A. Khan, F. Gul, S. Hussain, N. Ashraf, Toxicol. Ind. Health 31 (2013) 1281 (https://doi.org/10.1177/0748233713491813)
K. Srinivas, M.E. Rao, S.S. Rao, Ind. J Pharmacol. 32 (2000) 37 (https://ijp-online.com/temp/IndianJPharmacol32137-4643626_125356.pdf)
A. Kulkarni‑Almeidaa, A. Suthar, A. Goswami, R. Vishwakarma, V.S. Chauhan, A. Balakrishnan, Phytomedicine 15 (2008) 1079 (https://doi.org/10.1016/j.phymed.2008.04.013)
T. Machan, J. Korth, B. Liawruangrath, S. Liawruangrath, S. G., Thailand. Flavour Fragr. J. 21 (2006) 265 (https://doi.org/10.1002/ffj.1577)
J. S. Reddy, P. R. Rao, M. S. Reddy, J Ethnopharmacol. 79 (2002) 249 (https://doi.org/10.1016/S0378-8741(01)00388-9)
H. J. Walaa, M. N. Hamad, Plant Iraq. J. Pharm. Sci. 30 (2021) 158 (https://doi.org/10.31351/vol30iss2pp158-166)
P. R. Cheeke, J. Animal Sci. 66 (1988) 2343 (https://doi.org/10.2527/jas1988.6692343x)
N. Yassa, H. Farsam, A. Shafiee, A. Rustaiyan, Planta Med. 62 (1997) 583 (https://doi.org/10.1055/s-2006-957984)
M. A. Fayed, Phytomedicine Plus 1 (2021) 100036 (https://doi.org/10.1016/j.phyplu.2021.100036)
S. Bendeddouche, C. K. Bendeddouche, H. Benhaoua, Lett. Org. Chem. 18 (2021) 929 (https://doi.org/10.2174/1570178618666210901142356)
S. Park, R. J. Kazlauskas, J. Org. Chem. 66 (2001) 8395 (https://doi.org/10.1021/jo015761e)
V. Babushok, P. Linstrom, I. Zenkevich, J. Phys. Chem. Ref. Data 40 (2011) 043101 (https://doi.org/10.1063/1.3653552)
NIST17, Mass spectral library (NIST/EPA/NIH), National Institute of Standards and Technology, Gaithersburg, 2017 (DVD-ROM ISBN: 978-1-119-41223-6)
R. Adams, Identification of essential oil components by gas chromatography/mass spectrometry, 4th ed., Allured Publishing Corp., Carol Stream, IL, 2007 (ISBN-10: 1932633219)
Y. Zhou, D. Wu, P. Cai, G. Cheng, C. Huang, Y. Pan, Molecules 20 (2015) 7684 (https://doi.org/10.3390/molecules20057683)
M. H. Duan, M. Luo, C. J. Zhao, W. Wang, Y. G. Zu, D. Y. Zhang, X. H. Yao, Y. J. Fu, Separ. Purific. Technol. 107 (2013) 26 (https://doi.org/10.1016/j.seppur.2013.01.003)
W. Wang, Q. Li, Y. Liu, B. Chen, Ultrasonics Sonochem. 24 (2014) 13 (https://doi.org/10.1016/j.ultsonch.2014.10.009)
D.A. Fort, R.C. Remsing, R.P. Swatloski, P. Moyna, G. Moyna, R.D. Rogers, Green Chem. 9 (2007) 63 (https://doi.org/10.1039/B607614A)
D.W. Armstrong, L. He, Y.S. Liu, Anal. Chem. 71 (1999) 3873 (https://doi.org/10.1021/ac990443p)
I. Kilpeläinen, H. Xie, A. King, M. Granstrom, S. Heikkinen, D. S. Argyropoulos, J. Agric. Food Chem. 55 (2007) 9142 (https://doi.org/10.1021/jf071692e).