Anticancer activity of Schiff base ligand (E)-4-((5-chloro-2- -hydroxybenzylidene)amino)-1,5-dimethyl-2-phenyl-1H-pyrazol- -3(2H)-one and its Co(II), Cu(II) and Zn(II) metal complexes Scientific paper
Main Article Content
Abstract
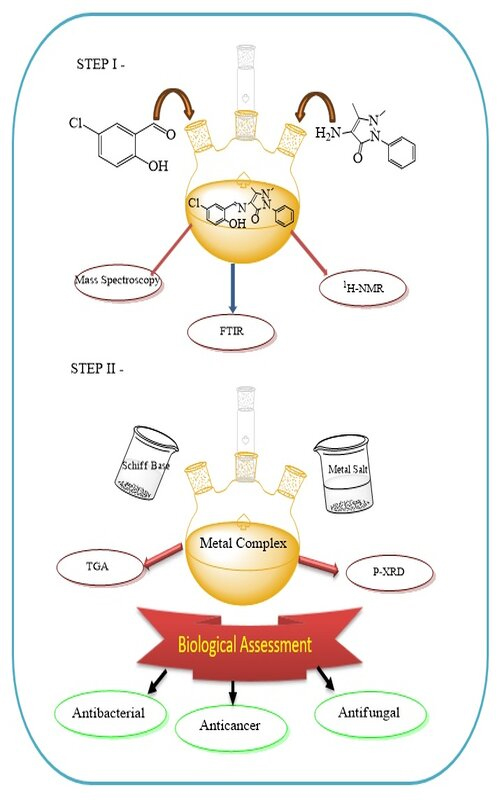
series of transition metal complexes of Mn(II), Co(II), Ni(II), Cu(II), Zn(II) and VO(II) have been prepared by using the Schiff base ligand (L) derived from 4-aminoantipyrine and 5-chlorosalicylaldehyde. The structural properties of Schiff base ligand were characterized by mass, FT-IR, UV–Vis, 1H-NMR spectroscopy, etc. Also, metal complexes were studied by P-
-XRD, elemental analysis, thermogravimetric studies along with various biological activities. The micro analytical data revealed that, the metal complexes have 1:1 stoichiometry composition of M:L. Generally, it is observed that prepared metal complexes show better antifungal, antibacterial and anticancer activities than its Schiff base ligand.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
M. Cinarli, C. YuksektepeAtaol, E. Cinarli, O. Idil, J. Mol. Struc. 1213 (2020) 128 (http://dx.doi.org/10.1016/j.molstruc.2020.128152)
F. I. Abouzayed, S. M. Emam, S. A. Abouel-Enein, J. Mol. Struc. 1216 (2020) 128 (http://dx.doi.org/10.1016/j.molstruc.2020.128314)
I. E. Leon, J. F. Cadavid-Vargas, A. L. Di Virgilio, S. B. Etcheverry, Curr. Med. Chem. 24 (2017) 2 (http://dx.doi.org/10.2174/0929867323666160824162546)
M. R. Gill, K. A. Vallis, Chem. Soc. Rev. 48 (2019) 540 (https://doi.org/10.1039/C8CS00641E)
A. Bijelic, M. Aureliano, A. Rompe, Angew. Chem. Int. Ed. 58 (2019) 2980(https://doi.org/10.1002/anie.201803868)
U. Ndagi, N. Mhlongo, M. E. Soliman, Drug Des. Dev. Ther. 11 (2017) 599 (https://doi.org/10.2147/DDDT.S119488)
T. Sakaeda, K. Kadoyama, Y. Okuno, Int. J. Med. Sci. 8 (2011) 487 (http://doi.org/10.7150/ijms.8.487)
N. J. Wheate, S. Walker, G. E. Craig, R. Oun, Dalton Trans. 39 (2010) 8113 (https://doi.org/10.1039/C0DT00292E)
E. Tisato, C. Marzano, M. Porchia, M. Pellei, C. Santini, Med. Res. Rev. 30 (2010) 708 (https://doi.org/10.1002/med.20174)
D. Denoyer, S. Masaldan, S. La Fontain, M. A. Cater, Metallomics 7 (2015) 1459 (https://doi.org/10.1039/c5mt00149h)
I. Mohanram, J. Meshram, Hindawi Publishing Corporation ISRN Organic Chemistry, 639392 (2014) (http://dx.doi.org/10.1155/2014/639392)
A A. Fadda, K. M. Elattar. J. Biosci. Med. 3 (2015) 114 (http://dx.doi.org/10.4236/jbm.2015.311015)
G. T. Zitouni, M. Sivaci, F. S. Kilic, K. Erol, Eur. J. Med. Chem. 36 (2001) 685 (https://doi.org/10.1016/S0223-5234(01)01252-1)
S. M. Sondhi, V. K. Sharma, N. Singhal, R. P. Verma, R. Shukla, R. Raghubir, M. P. Dubey, Phosphorous, Sulfur, Silicon Relat. Elem. 156 (2000) 21 (https://doi.org/10.1080/10426500008044991)
H. M. Y. Al-Labban, H. M. Sadiq, A. A. J. Aljanaby. J. Phys.: Conf. Ser. 1294 (2019) 052007 (https://doi.org/10.1088/1742-6596/1294/5/052007)
M. Gowri, B. Saranya, S. Athimoolam, Ind. J. Chem., B 60 (2021) 1110 (http://nopr.niscair.res.in/handle/123456789/58188)
E. S. H. El ashry, L. F. Awad, E. I. Ibrahim, O. Kh. Bdeewy, China J. Chem. 25 (2007) 520 (https://doi.org/10.1002/cjoc.200790107)
A. Fadda, K. M. Elatter, Am. J. Org. Chem. 2 (2012) 52 (http://dx.doi.org/10.5923/j.ajoc.20120203.03)
Y.-X. Zou, X. Feng, Z.-Y. Chu, W.-H. Liu, X.-D. Zhang, J.-B. Ba, Regulat. Toxicol. Pharmacol. 103 (2019) 34 (https://doi.org/10.1016/j.yrtph.2019.01.018)
M. Verleyeu, I. Heulardand, J. M. Gillardin, Pharmacol. Res. 41 (2000) 539 (http://dx.doi.org/10.1006/phrs.1999.0619)
R. K. Mishra, B. G. Thakur, Asian J. Chem. 27 (2015) 860 (http://dx.doi.org/10.14233/ajchem.2015.17144)
Y. N. Bharate, M. A. Sakhare, S. B. Jadhav, S. D. Naikade, Rasayan J. Chem. 14 (2021) 479 (http://dx.doi.org/10.31788/RJC.2021.1416097).
Y. X. Sun, Acta Crystallogr., E 62 (2006) 05858 (http://doi.org/10.1107/S1600536806049348)
B. Anupama, M. Padmaja, C. Gyana Kumari, E-J. Chem. 9 (2012) 389. (http://dx.doi.org/10.1155/2012/291850)
R. Ramesh, S. Maheshwaram, J. Inorg. Biochem. 96 (2003) 457 (https://doi.org/10.1016/S0162-0134(03)00237-X)
S. A. Ali, A. A. Soliman, M. M. Aboaly, R. M. Ramadan, J. Coord. Chem. 55 (2013) 1161 (http://dx.doi.org/10.1080/0095897021000023509)
K. Singh, Y. Kumar, P. Puri, C. Sharma, K. R. Aneja, Arab. J. Chem. 10 (2017) 978 (https://doi.org/10.1016/j.arabjc.2012.12.038)
M. A. Sakhare, S. A. Khillare, M. K. Lande, B. R. Arbad, Adv. Appl. Sci. Res. 4 (2013) 94 (https://www.primescholars.com/articles/synthesis-characterisation-and-antimicrobial-studies-on-laiii-ceiii-andpriii-complexes-with-a-tetraaza-macrocyclic-ligand.pdf)
T. J. Saritha, P. Metilda, J. Saudi Chem. Soc. 25 (2021) 101245 (https://doi.org/10.1016/j.jscs.2021.101245)
P. Jayaseelan, E. Akila, M. Usha Rani, R. Rajavel, J. Saudi Chem. Soc. 20 (2016) 625(http://dx.doi.org/10.1016/j.jscs.2013.07.001)
A. A. S. Al-Hamdani, A. M. Balkhi, A. Falah. S. A. Shaker, J. Chil. Chem. Soc. 60 (2015) 2774 (http://dx.doi.org/10.4067/S0717-97072015000100003)
Y. N. Bharate, K. B. Sakhare, S. A. Surwase, M. A. Sakhare, Heterocycl. Lett. 13 (2023) 45 (https://www.heteroletters.org/issue131/Paper-5.pdf)
A. Aghao, D. Janrao, S. Janrao, M. Farooqui, J. Pharma Res. 5 (2016) 94 (https://jprinfo.com/storage/live/2020-04-05%2000_02_574.Arvind%20Aghao.pdf)
Y. N. Bharate, K. B. Sakhare, S. J. Chavan, M. A. Sakhare, Int. J. Sci. Res. Sci. Tech. 9 (2022) 116 (https://ijsrst.com/IJSRST22291617)
L. K. Dammu, S. K. Nara, V. Venkata, J. Nimmagadda, S. Kalluru., J. Adv. Sci. Res. 14 (2023) 35-39 (https://doi.org/10.55218/JASR.202314105)
L. H. Abdel-Rahman, A. M. Abu-Dief, E. F. Newair, S. K. Hamdan, J. Photochem. Photobiol. 18 (2016) 160 (http://dx.doi.org/10.1016/j.jphotobiol.2016.03.040)
A. M. Abu-Dief, L. A. Nassr, J. Iran. Chem. Soc. 12 (2015) 943 (https://doi.org/10.1007/s13738-014-0557-9)
P. S. Mane, S. G. Shirodkar, B. R. Arbad, T. K. Chondhekar, Ind. J. Chem., A 40 (2001) 648 (http://nopr.niscpr.res.in/handle/123456789/21060)
P. V. Rao, A. V. Narasaiah, Ind. J. Chem., A 42 (2003) 1896 (http://nopr.niscpr.res.in/handle/123456789/20716)c
H. Laila, A. Rahman, A. M. Abu-Dief, R. M. El-Khatib, S. M. Abdel-Fatah, Photochem. Photobiol. 69 (2016) 140 (http://doi/10.1016/j.jphptobiol.2016.06.052).