Simultaneous determination of emtricitabine and tenofovir disoproxil fumarate in pharmaceutical preparations using spectrophotometric, chemometric and chromatographic methods Scientific paper
Main Article Content
Abstract
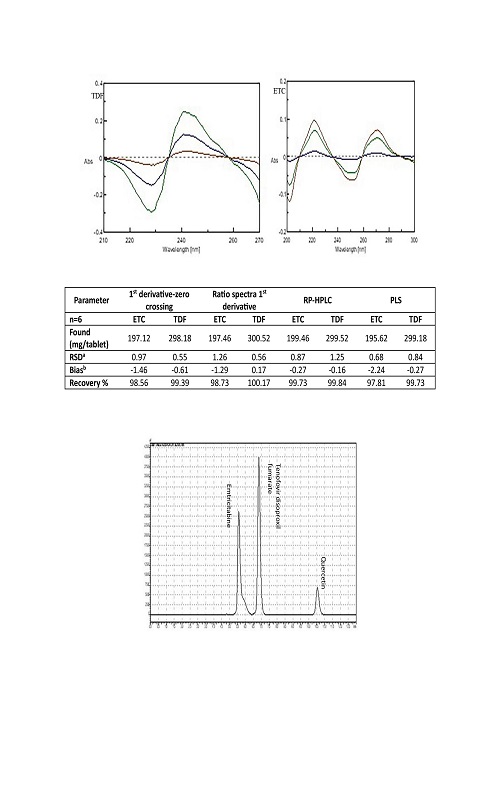
Simple, accurate and sensitive spectrophotometric, chemometric and chromatographic methods were used for the simultaneous determination of emtricitabine (ETC) and tenofovir disoproxil fumarate (TDF) in tablets. In 1st derivative spectrophotometry, the first derivative spectra of the solution of ETC and TDF in water were recorded as ∆λ = 4 nm and the first derivative absorances were measured at the zero-crossing points at 297.3 and 281.2 nm for ETC and TDF, respectively. In ratio of the 1st derivative spectrophotometry measurements were recorded at 239.0 and 270.2 nm for ETC and TDF, respectively. Then analytical signals were measured at the wavelengths corresponding to either maximum or minimum for both drugs. For these spectrophotometric methods Beer’s law is obeyed in the concentration range of 2–15 µg mL-1 for both drugs. As chemometric method, the PLS technique was used. In chromatographic method, the separation was achieved on a C18 column with DAD (262 nm) and isocratic elution of methanol, acetonitrile and 0.1 % orthophosphoric acid in the volume ratio of 40:40:20, respectively, containing the mobile phase. The mean recovery and the relative standard deviation of the methods were found as 97.51–100.17 % and 0.55–1.26 % respectively. All these methods were statistically compared, and they were successfully applied to a pharmaceutical preparation.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
L. M. Bang, L. J. Scott, Drugs 63 (2003) 2413 (http://dx.doi.org/10.2165/00003495-200363220-00003)
C. Orkin, J. M. Llibre, S. Gallien, A. Antinori, G. Behrens, A. Carr, HIV Medicine 19 (2018) 18 (http://dx.doi.org/10.1111/hiv.12534)
C. Wassner, N. Bradley, Y. Lee, J. Int. Assoc. Providers AIDS Care 19 (2020) 1 (http://dx.doi.org/10.1177/2325958220919231)
K. A. Lyseng-Williamson, N. A. Reynolds, G. L. Plosker, Drugs 65 (2005) 413 (http://dx.doi.org/10.2165/00003495-200565030-00006)
C. Runja, P. R. Kumar, S. R. Avanapu, J. Chromatogr. Sci. 54 (2016) 759 (http://dx.doi.org/10.1093/chromsci/bmw004)
B. Lavanya, P. Hariprasad, A. Venkatapraveen, D.P. Lakshmi, M. Ramireddy, Pharm. Lett. 4 (2012) 99 (https://www.scholarsresearchlibrary.com/abstract/simultaneous-estimation-of-emtricitabine-and-tenofovir-disproxil-fumerate-byrnhplc-method-5960.html)
R. R. Jampala, V. K. Kumar, A. R. Nemala. Pharm. Methods 5 (2014) 7 (http://dx.doi.org/10.5530/phm.2014.1.2)
K. Anandakumara, G. Abiramia, S. Murugana, B. Ashokb, J. Anal. Chem. 68 (2013) 815 (http://dx.doi.org/10.1134/S1061934813090025)
M. Yadav, T. Mishra, P. Singhal, S. Goswami, P. S. Shrivastav, J. Chromatogr. Sci. 47(2009) 140 (http://dx.doi.org/10.1093/chromsci/47.2.140)
P. Tiwari, R. Yadav, K. Avinash, V. Vaidya, P.A. Sathe, D. Gangrade Anal. Chem. Ind. J. 9 (2010) 247 (https://www.tsijournals.com/articles/development-and-validation-of-uplc-method-for-emtricitabine-tenofovir-and-efavirenz-in-pharmaceutical-prepartion.pdf)
M. H. AbdelHay, A. A. Gazy, R. A. Shaalan, H. K. Ashour, J. Spectrosc. 2013 (2013) 937409 (https://doi.org/10.1155/2013/937409)
J. Saminathan, T. Vetrichelvan, Bangladesh Pharm. J. 19 (2016) 114 (https://doi.org/10.3329/bpj.v19i1.29247)
B. Lavanya, P. Hariprasad, A. Venkatapraveen, D. Prasannalakshmi, Int. J. Res. Pharm. Life Sci. 3 (2012) 104 (https://irjponline.com/admin/php/uploads/1542_pdf.pdf)
S. Venkatesan, N. Kannappan, Int. Scholarly Res. Notices 2014 (2014) 541727 (https://doi.org/10.1155/2014/541727)
H. K. Ashour, T. S. Belal, Arab. J. Chem. 10 (2017) S1741 (https://doi.org/10.4103/0250-474X.51951)
M. Joshi, A.P. Nikalje, M. Shahed, M. Dehghan, Ind. J. Pharm. Sci. 71 (2009) 95 (https://doi.org/10.4103/0250-474X.51951)
M.K. Matta, N.R. Pilli, S. Rao J.V.L.NA, Acta Chromatogr. 1 (2015) 27 (https://doi.org/10.1556/achrom.27.2015.1.3)
M. Takahashi, Y. Kudaka, N. Okumura, A. Hirano, K. Banno, T. Kaneda, Biol. Pharm. Bull. 30 (2007)1784 https://www.jstage.jst.go.jp/article/bpb/30/9/30_9_1784/_pdf
C. Bennetto-Hood, M.C. Long, E.P. Acosta, Rapid Commun. Mass Spectrom. 21 (2007) 2087 (https://doi.org/10.1002/rcm.3056)
S. Thomas, P. Shanmugasundramb, Ind. Drugs 55 (2018) 44 (https://doi.org/10.53879/id.55.01.10819)
V. D. Singh, V. K. Singh, S. J. Daharwal, J. AOAC Int. (2023) qsad067 (https://doi.org/10.1093/jaoacint/qsad067)
B. Morelli, J. Pharm. Biomed. Anal. 13 (1995) 219 (https://doi.org/10.1016/0731-7085(95)01250-O)
V.P. Choudhari, S. Ingale, S.R. Gite, D.D. Tajane, V.G. Modak, A. Ambekar, Pharm. Methods 2 (2011) 47 (https://doi.org/10.4103/2229-4708.81096)
M. G. Caglayan, I. M. Palabiyik, M. Bor, F. Onur, Chem. Papers 65 (2011) 754 (https://doi.org/10.2478/s11696-011-0078-2)
E. Dinc¸ I. M. Palabiyik, O. Üstundag, F. Yurtsever, F. Onur, J. Pharm. Biomed. Anal. 28 (2002) 591 (https://doi.org/10.1016/S0731-7085(01)00694-X)
I. M. Palabiyik, F. Onur, C. Yardimci, N. Özaltin, Quim. Nova 31 (2008) 1121 (https://www.scielo.br/j/qn/a/CkbxNDw4jqQx8TNzNppFJVy/)
S. Wold, M. Sjöström, L. Eriksson, Chemomet. Intell. Labor. Systems 58 (2001) 109 (https://doi.org/10.1016/S0169-7439(01)00155-1)
R. G. Brereton, Chemometrics, Data Analysis for the Laboratory and Chemical Plant, John Wiley & Sons, New York, 2002 (computer program)
ICH, Harmonized tripartite guideline validation of analytical procedures: text and methodology Q2(R1) (assessed August 31, 2023) (https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf)
ICH, Harmonized Tripartite Guideline, on validation of analytical procedures Q2(R2), ICH Steering Committee, 2022 (assessed August 31, 2023) (https://database.ich.org/sites/default/files/ICH_Q2-R2_Document_Step2_Guideline_2022_0324.pdf)