In vitro anticancer studies of a small library of cyclic lipopeptides against the human cervix adenocarcinoma HeLa cells Scientific paper
Main Article Content
Abstract
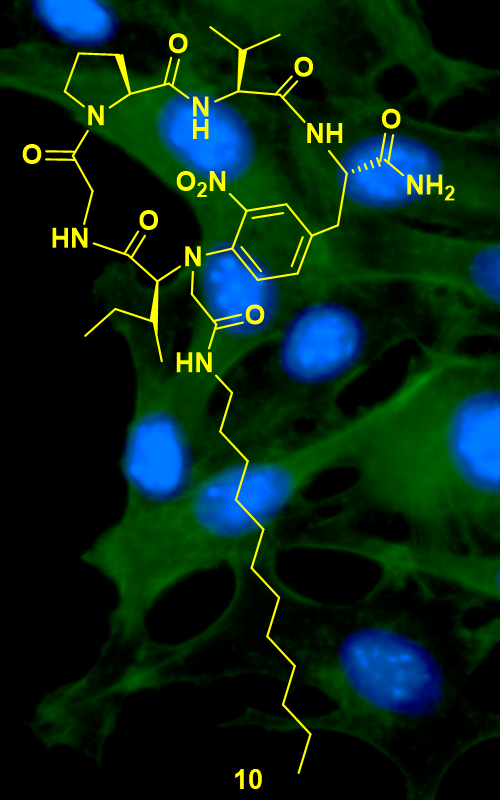
Various cyclic lipopeptides (CLPs, 23 compounds) were tested for their antitumor potential against human cervix adenocarcinoma HeLa cells. From the fast screening (tested concentrations: 0.01 and 10 µM) compound 10 ((12S,6S,10S,13S)-6-((R)-sec-butyl)-7-(2-(dodecylamino)-2-oxoethyl)-13-isopropyl-82-nitro-2,5,12,15-tetraoxo-4,7,11,14-tetraaza-1(1,2)-pyrrolidina-8(1,4)-benzenacyclopentadecaphane-10-carboxamide) was identified as active against HeLa cell line. The MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and CV (crystal violet) assays revealed at least five times higher cytotoxic potential of 10 (IC50 = 12.3±1.8 µM, MTT; 9.4±1.5 µM; CV) in comparison to control drug natural occurring CLP surfactin (IC50 = 64.9±0.8 µM, MTT; 76.2±1.6 µM; CV). The cell cycle analysis performed by DAPI (4',6-diamidino-2-phenylindole) assay indicated the involvement of apoptosis in HeLa cell death upon treatment with 10, which was confirmed by apoptosis assay (annexin V/PI). Furthermore, during this process caspase activation could be detected (ApoStat assay, immunocytochemistry caspase-3 analysis). The flow cytometry analysis did not display induction of autophagy as a possible death mechanism in HeLa cells upon 10 treatment. The current findings could be used to design more effective CLPs based on 10 structure as potential anticancer agents.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200116
References
R. L. Siegel, K. D. Miller, N. S. Wagle, A. Jemal, Ca Cancer J. Clin. 73 (2023) 17 (https://doi.org/10.3322/caac.21763 )
M. Malvezzi, C. Santucci, P. Boffetta, G. Collatuzzo, F. Levi, C. La Vecchia, E. Negri, Ann. Oncol. 34 (2023) 410 (https://doi.org/10.1016/j.annonc.2023.01.010 )
M. Muscaritoli, J. Arends, P. Bachmann, V. Baracos, N. Barthelemy, H. Bertz, F. Bozzetti, E. Hütterer, E. Isenring, S. Kaasa, Clin. Nutr. 40 (2021) 2898 (https://doi.org/10.1016/j.clnu.2021.02.005 )
S. Liu, Q. Sun, X. Ren, J. Hematol. Oncol. 16 (2023) 38 (https://doi.org/10.1186/s13045-023-01430-8 )
S. L. Gupta, S. Basu, V. Soni, R. K. Jaiswal, Mol. Biol. Rep. 49 (2022) 9903 (https://doi.org/10.1007/s11033-022-07525-8 )
K. O’Brien, K. Ried, T. Binjemain, A. Sali, Cancers 14 (2022) 5933 (https://doi.org/10.3390/cancers14235933 )
Y. F. Mustafa, Appl. Nanosci. 13 (2023) 1907 (https://doi.org/10.1007/s13204-021-01872-x )
S. Motyka, K. Jafernik, H. Ekiert, J. Sharifi-Rad, D. Calina, B. Al-Omari, A. Szopa, W. C. Cho, Biomed. Pharmacother. 158 (2023) 114145 (https://doi.org/10.1016/j.biopha.2022.114145 )
Z. Breijyeh, R. Karaman, Antibiotics 12 (2023) 628 (https://doi.org/10.3390/antibiotics12030628 )
M. C. Morejón, A. Laub, G. N. Kaluđerović, A. R. Puentes, A. N. Hmedat, A. J. Otero-
-González, D. G. Rivera, L. A. Wessjohann, Org. Biomol. Chem. 15 (2017) 3628 (https://doi.org/10.1039/c7ob00459a )
S. Lai, Q. Zhang, L. Jin, Antibiotics 12 (2022) 42 (https://doi.org/10.3390/antibiotics12010042 )
S. Singh, R. A. Sequeira, P. Kumar, V. A. Ghadge, P. Vaghela, A. K. Mohanty, A. Ghosh, K. Prasad, P. B. Shinde, ACS Omega 7 (2022) 46646 (https://doi.org/10.1021/acsomega.2c05587 )
D. P. Fewer, J. Jokela, L. Heinilä, R. Aesoy, K. Sivonen, T. Galica, P. Hrouzek, L. Herfindal, Physiol. Plant. 173 (2021) 639 (https://doi.org/10.1111/ppl.13484 )
J. Steigenberger, Y. Verleysen, N. Geudens, A. Madder, J. C. Martins, H. Heerklotz, Biophys. J. 122 (2023) 950 (https://doi.org/10.1016/j.bpj.2022.07.033 )
C. Wan, X. Fan, Z. Lou, H. Wang, A. Olatunde, K. R. Rengasamy, Crit. Rev. Food Sci. Nutr. 62 (2022) 7976 (https://doi.org/10.1080/10408398.2021.1922355 )
A. Evidente, Int. J. Mol. Sci. 23 (2022) 12342 (https://doi.org/10.3390/ijms232012342 )
J. G. Tank, R. V. Pandya, Peptides 155 (2022) 170836 (https://doi.org/10.1016/j.peptides.2022.170836 )
A. Théatre, C. Cano-Prieto, M. Bartolini, Y. Laurin, M. Deleu, J. Niehren, T. Fida, S. Gerbinet, M. Alanjary, M. H. Medema, Front. Bioeng. Biotechnol. 9 (2021) 623701 (https://doi.org/10.3389/fbioe.2021.623701 )
A. Rosier, M. Pomerleau, P. B. Beauregard, D. A. Samac, H. P. Bais, Plants 12 (2023) 1007 (https://doi.org/10.3390/plants12051007 )
J.-F. Liu, S. M. Mbadinga, S.-Z. Yang, J.-D. Gu, B.-Z. Mu, Int. J. Mol. Sci. 16 (2015) 4814 (https://doi.org/10.3390/ijms16034814 )
Y.-S. Wu, S.-C. Ngai, B.-H. Goh, K.-G. Chan, L.-H. Lee, L.-H. Chuah, Front. Pharmacol. 8 (2017) 761 (https://doi.org/10.3389/fphar.2017.00761 )
T. T. T. Vo, Y. Wee, H. C. Cheng, C. Z. Wu, Y. L. Chen, V. P. Tuan, J. F. Liu, W. N. Lin, I. T. Lee, Oral Dis. 29 (2023) 528 (https://doi.org/10.1111/odi.13950 )
M. C. Morejón, New Multicomponent Strategies to Cyclic Lipopeptides, Universitäts-und Landesbibliothek Sachsen-Anhalt, Halle (Saale), 2018 (https://doi.org/10.1515/bd.2003.37.1.41 )
T. Krajnović, N. Đ. Pantelić, K. Wolf, T. Eichhorn, D. Maksimović-Ivanić, S. Mijatović, L. A. Wessjohann, G. N. Kaluđerović, Materials 15 (2022) 5028 (https://doi.org/10.3390/ma15145028 )
I. Morgan, L. A. Wessjohann, G. N. Kaluđerović, Cells 11 (2022) 168 (https://doi.org/10.3390/cells11010168)
S. B. Kntayya, M. D. Ibrahim, N. Mohd Ain, R. Iori, C. Ioannides, A. F. Abdull Razis, Nutrients 10 (2018) 718 (https://doi.org/10.3390/nu10060718 )
N. Ganesan, S. Ronsmans, P. Hoet, Heliyon 9 (2023) e19242 (https://doi.org/10.1016/j.heliyon.2023.e19242 )
N. Yusof, N. M. Yasin, R. Yousuf, A. A. Wahab, S. A. Aziz, Bangladesh J. Med. Sci. 21 (2022) 626 (https://doi.org/10.3329/bjms.v21i3.59577 )
I. Canga, P. Vita, A.I. Oliveira, M. Á. Castro, C. Pinho, Molecules 27 (2022) 4989 (https://doi.org/10.3390/molecules27154989 )
R. Gómez-Bombarelli, E. Calle, J. Casado, J. Org. Chem. 78 (2013) 6868 (https://doi.org/10.1021/jo400258w )
K. Liu, Y. Sun, M. Cao, J. Wang, J. R. Lu, H. Xu, Curr. Opin. Colloid Interface Sci. 45 (2020) 57 (https://doi.org/10.1016/j.cocis.2019.12.005 )
M. Delgado, R. R. Rainwater, B. Heflin, A. Urbaniak, K. Butler, M. Davidson, R. M. Protacio, G. Baldini, A. Edwards, M. R. Reed, J. Biol. Chem. 298 (2022) 101939 (https://doi.org/10.1016/j.jbc.2022.101939 )
M. Maruoka, P. Zhang, H. Mori, E. Imanishi, D. M. Packwood, H. Harada, H. Kosako, J. Suzuki, Mol. Cell 81 (2021) 1397 (https://doi.org/10.1016/j.molcel.2021.02.025 )
J. Debnath, N. Gammoh, K. M. Ryan, Nat. Rev. Mol. Cell Biol. 24 (2023) 560
(https://doi.org/10.1038/s41580-023-00585-z )
Y. Zhou, H. Manghwar, W. Hu, F. Liu, Int. J. Mol. Sci. 23 (2022) 7301 (https://doi.org/10.3390/ijms23137301 )
G. N. Kaluđerović, V. M. Đinović, Z. D. Juranić, T. P. Stanojković, T. J. Sabo, J. Inorg. Biochem. 99 (2005) 488 (https://doi.org/10.1016/j.jinorgbio.2004.10.025)
Z. T. A. Ribeiro, E. Machado-Ferreira, L. F. Guimarães, J. Cavaleiro, A. M. A. Britto, N. Redua, L. M. P. de Souza, A. S. Pimentel, P. H. S. Picciani, O. N. Oliveira, C. B. Barreto, C. A. G. Soares, Colloids Surfaces, A 626 (2021) 126984 (https://doi.org/10.1016/j.colsurfa.2021.126984)
R. Csuk, L. Heller, B. Siewert, A. Gutnov, O. Seidelmann, V. Wendisch, Bioorg. Med. Chem. Lett. 24 (2014) 4011 (https://doi.org/10.1016/j.bmcl.2014.06.021).