Pressure oxidative leaching of copper concentrate
Main Article Content
Abstract
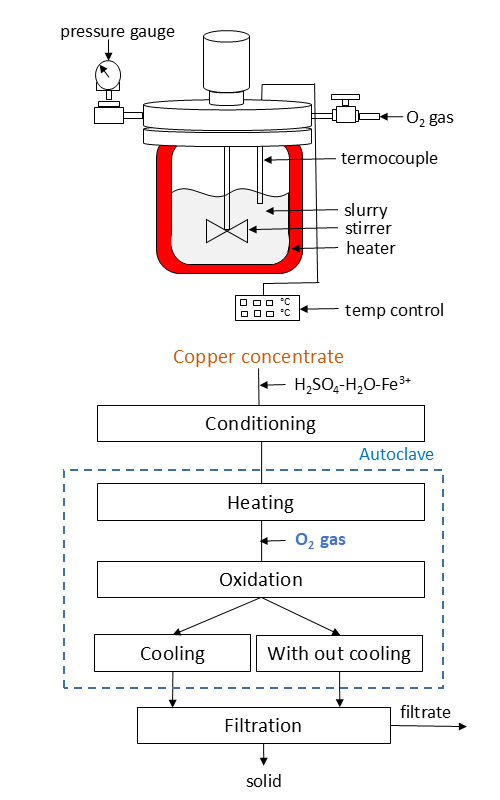
This study investigated the oxidative leaching of copper concentrate using a mixture of ferric ions and sulfuric acid solutions. We examined the effects of various parameters, including temperature, initial sulfuric acid concentration, and slurry filtration conditions. At lower temperatures (150 °C), the leaching process resulted in the elemental sulfur and jarosite minerals formed in the solid residue. In contrast, at higher temperatures (190 °C), the solid residue contained jarosite and hematite, the most elemental sulfur-oxidizing to sulfuric acid. Under optimal conditions, a leaching temperature of 190 °C, a concentrate-to-leaching solvent (Fe³⁺ 5 g l-1 and H₂SO₄ 50 g l-1) ratio of 1:8, an oxygen pressure of 1.0 MPa, and a solid phase particle size of less than 20 μm the dissolution rate of copper reached 98% after three hours. When the sulfuric acid concentration was increased from 30 g l-1 to 100 g l-1, the amount of copper was increased from 40 g l-1 to 48 g l-1. Furthermore, rapid filtering of the leaching solution without cooling helped retain most of the iron in the solid phase, resulting in a relatively pure solution. The solid residue was analyzed using X-ray diffraction (XRD) and scanning electron microscopy (SEM).
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
2021/08, https://mrpam.gov.mn/public/pages/169/2021.08.stat.report.mon.pdf (accessed: april 2025)
N. Tumen-Ulzii, B. Gunchin, J. Serbian Chem. Soc. 88 (2023) 1149–1160 (https://doi.org/10.2298/JSC230114057T)
S. Wang, Jom 57 (2005) 48–51 (https://doi.org/10.1007/s11837-005-0252-5)
A. A. Baba, K. I. Ayinla, F. A. Adekola, M. K. Ghosh, O. S. Ayanda, R. B. Bale, A. R. Sheik, S. R. Pradhan, Int. J. Min. Eng. Miner. Process. 1 (2012) 1–16 (https://doi.org/10.5923/j.mining.20120101.01)
J. Cháidez, J. Parga, J. Valenzuela, R. Carrillo, I. Almaguer, Metals (Basel). 9 (2019) 189 (https://doi.org/10.3390/met9020189)
B. Han, B. Altansukh, K. Haga, Y. Takasaki, A. Shibayama, J. Sustain. Metall. 3 (2017) 528–542 (https://doi.org/10.1007/s40831-017-0135-3)
K. Takatori, H. Kato, A. Yoshimura, Y. Matsuno, Mining, Metall. Explor. 38 (2021) 1477–1485 (https://doi.org/10.1007/s42461-021-00400-3)
M. Sokić, B. Marković, S. Stanković, Ž. Kamberović, N. Štrbac, V. Manojlović, N. Petronijević, Metals (Basel). 9 (2019) 1173 (https://doi.org/10.3390/met9111173)
F. Saloojee, F. K. Crundwell, J. South African Inst. Min. Metall. 116 (2016) 517–524 (https://doi.org/10.17159/2411-9717/2016/v116n6a5)
S. Heguri, S. Asano, A. Idegami, J. MMIJ 131 (2015) 470–475 (https://doi.org/10.2473/journalofmmij.131.470)
S. Matuska, K. Ochromowicz, T. Chmielewski, Physicochem. Probl. Miner. Process. 54 (2018) 781–792 (https://bibliotekanauki.pl/articles/110420.pdf)
E. Uzun, M. Zengin, Ý. Atỳlgan, Mater. Tehnol. 50 (2016) 395–401 (https://doi.org/10.17222/mit.2015.091)
E. M. Córdoba, J. A. Muñoz, M. L. Blázquez, F. González, A. Ballester, Hydrometallurgy 93 (2008) 81–87 (https://doi.org/10.1016/j.hydromet.2008.04.015)
J. Gega, W. Walkowiak, Physicochem. Probl. Miner. Process. 46 (2011) 155 (https://www.dbc.wroc.pl/Content/10110/46_2011.pdf)
K. J. Nyembwe, E. Fosso-Kankeu, F. Waanders, M. Mkandawire, Trans. Nonferrous Met. Soc. China (English Ed. 31 (2021) 2139–2152 (https://doi.org/10.1016/S1003-6326(21)65644-3)
S. J. Petrović, G. D. Bogdanović, M. M. Antonijević, M. Vukčević, R. Kovačević, Metals (Basel). 13 (2023) 1818 (https://doi.org/10.3390/met13111818)
S. J. Petrović, G. D. Bogdanović, M. M. Antonijević, Trans. Nonferrous Met. Soc. China (English Ed). 28 (2018) 1444–1455 (https://doi.org/10.1016/S1003-6326(18)64788-0)
H. B. Zhao, M. H. Hu, Y. N. Li, S. Zhu, W. Q. Qin, G. Z. Qiu, J. Wang, Trans. Nonferrous Met. Soc. China (English Ed.) 25 (2015) 303–313 (https://doi.org/10.1016/S1003-6326(15)63605-6)
Y. Xing, C. Wei, Z. Deng, X. Li, M. Li, Sci. Rep. 14 (2024) 24490 (https://doi.org/10.1038/s41598-024-75857-5)
C. Li, Z. Deng, C. Wei, G. Fan, X. Li, M. Li, Y. Wang, Hydrometallurgy 178 (2018) 294–300 (https://doi.org/10.1016/j.hydromet.2018.05.012).