Georgian bentonite clay–polymer system as a carrier for volatile oil in topical applications Scientific paper
Main Article Content
Abstract
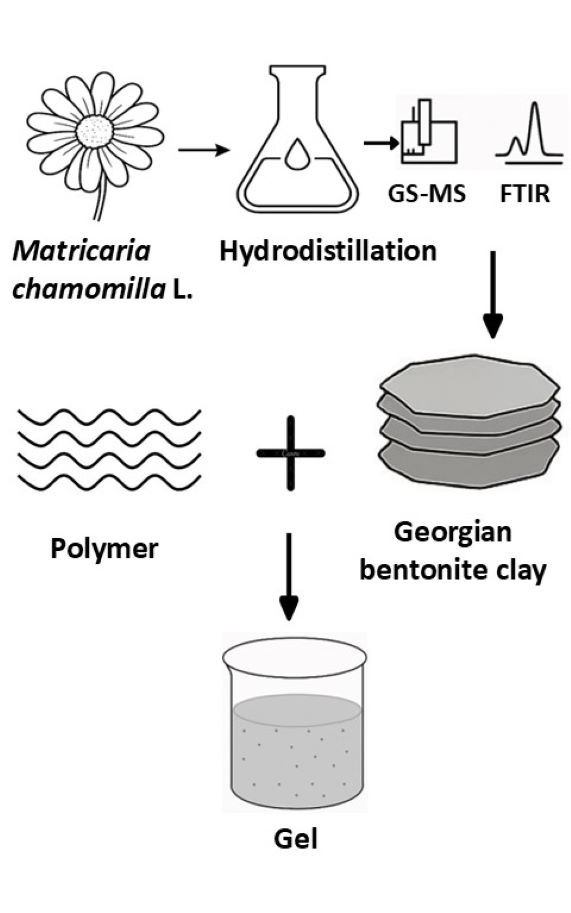
This study focuses on developing a polymer–clay hybrid system using a Georgian bentonite clay (Tikha-Ascane, TA) and Carbopol (CA) as a carrier for an essential oil (EO) derived from Matricaria chamomilla L. cultivated in East Georgia. EO was extracted via hydro distillation and characterized using GC–MS and FTIR techniques. The CA–TA and CA–TA–EO formulations were evaluated for key physicochemical parameters including pH, viscosity, rheology, spreadability, compatibility, moisture loss, uniformity and stability. The EO yield was 0.3 % from air-dried plant material. The main components were bisabolol oxide A (38.2 %), α-bisabolol oxide B (12.91 %), (cis)-β-farnesene (11.8 %), α-bisabolol (8.83 %), spathulenol (2.27 %), chamazulene (1.99 %), cis-ene-yne-dicycloether (6.51 %) and β-copaene (0.38 %). These results suggest that the local chamomile chemotype is rich in bisabolol oxide A. The optimized hydrogel formulation was homogeneous, stable and demonstrated favorable rheological properties, making it suitable for topical application. Chromatographic analysis confirmed the successful incorporation of the EO into the gel, which achieved an encapsulation efficiency of 58.34 %. Overall, the study confirms the compatibility of Georgian bentonite clay with the CA polymer, forming an effective matrix for volatile oil delivery in semisolid dosage forms.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
D. P. de Sousa, R. O. S. Damasceno, R. Amorati, H. A. Elshabrawy, R. D. de Castro, D. P. Bezerra, V. R. V. Nunes, R. C. Gomes, T. C. Lima, Biomolecules 13 (2023) 1144 (https://doi.org/10.3390/biom13071144)
A. El Mihyaoui, J. C. G. Esteves da Silva, S. Charfi, M. E. Candela Castillo, A. Lamarti, M. B. Arnao, Life 12 (2024) 479 (https://doi.org/10.3390/life12040479)
G. Ren, G. Ke, R. Huang, Q. Pu, J. Zhao, Q. Zheng, M. Yang, Sci. Rep. 12 (2022) 8153 (https://doi.org/10.1038/s41598-022-11692-w)
J. N. Saucedo-Zuñiga, S. Sánchez-Valdes, E. Ramírez-Vargas, L. Guillen, L. F. Ramos-
-deValle, A. Graciano-Verdugo, J. A. Uribe-Calderón, M. Valera-Zaragoza, T. Lozano-
-Ramírez, J. A. Rodríguez-González, J. J. Borjas-Ramos, J. D. Zuluaga-Parra, Microporous Mesoporous Mater. 316 (2021) 110882 (https://doi.org/10.1016/j.micromeso.2021.110882)
L. H. Oliveira, I. S. de Lima, E. R. S. da Neta, S. G. de Lima, P. Trigueiro, J. A. Osajima, E. C. da Silva-Filho, M. Jaber, M. G. Fonseca, Appl. Clay Sci. 245 (2023) 107158 (https://doi.org/10.1016/j.clay.2023.107158)
A. Giannakas, I. Tsagkalias, D. S. Achilias, A. Ladavos, Appl. Clay Sci. 146 (2017) 362 (https://doi.org/10.1016/j.clay.2017.06.018)
F. Sorouri, P. Azimzadeh Asiabi, P. Hosseini, A. Ramazani, S. Kiani, T. Akbari, M. Sharifzadeh, M. Shakoori, A. Foroumadi, L. Firoozpour, M. Amin, M. Khoobi, Polym. Bull. 80 (2023) 5101 (https://doi.org/10.1007/s00289-022-04306-y)
M. Chelu, Gels 10 (2024) 636 (https://doi.org/10.3390/gels10100636)
I. G. Kutateladze, Tikha-Ascane for medical purpose, Gruzmedgiz, Tbilisi, 1955, p. 40
L. Tsiklauri, I. Dadeshidze, G. Tsagareishvili, Bull. Georgian Natl. Acad. Sci. 150 (1994) 262 (ISSN 0132-1447)
N. Tsivelika, E. Sarrou, K. Gusheva, C. Pankou, T. Koutsos, P. Chatzopoulou, A. Mavromatis, Biochem. Syst. Ecol. 80 (2018) 21 (https://doi.org/10.1016/j.bse.2018.06.001)
R. Ensandoost, H. Izadi-Vasafi, H. Adelnia, J. Macromol. Sci. Phys. 61 (2021) 225 (https://doi.org/10.1080/00222348.2021.1999043)
N. Ullah, A. Amin, A. Farid, S. Selim, S. A. Rashid, M. I. Aziz, S. H. Kamran, M. A. Khan, N. Rahim Khan, S. Mashal, M. M. Hasan, Gels 9 (2023) 252 (https://doi.org/10.3390/gels9030252)
M. Singh, J. Kanoujia, P. Parashar, M. Arya, C. B. Tripathi, V. R. Sinha, S. K. Saraf, S. A. Saraf, Drug Deliv. Transl. Res. 8 (2018) 591 (https://doi.org/10.1007/s13346-018-0489-5)
B. Siddiqui, A. U. Rehman, I. U. Haq, N. M. Ahmad, N. Ahmed, J. Microencapsul. 37 (2020) 595 (https://doi.org/10.1080/02652048.2020.1829140)
I. Ikeda-Ogata, K. Yamasaki, K. Yokomizo, T. Ikeda, H. Seo, Curr. Top. Phytochem. 17 (2021) 63 (http://www.researchtrends.net/tia/article_pdf.asp?in=0&vn=17&tid=24&aid=6834)
Ö. Süfer, F. Bozok, J. Therm. Anal. Calorim. 140 (2020) 253 (https://doi.org/10.1007/s10973-019-08829-x)
S. Agatonovic-Kustrin, P. Ristivojevic, V. Gegechkori, T. M. Litvinova, D. W. Morton, Appl. Sci. 10 (2020) 7294 (https://doi.org/10.3390/app10207294)
L. Ciko, A. Andoni, F. Ylli, E. Plaku, K. Taraj, J. Int. Environ. Appl. Sci. 11 (2016) 154 (https://dergipark.org.tr/tr/download/article-file/571496)
M. I. Morar, F. Fetea, A. M. Rotar, M. Nagy, C. A. Semeniuc, Bull. UASVM Food Sci. Technol. 74 (2017) 37 (https://doi.org/10.15835/buasvmcn-fst:12634)
H. Schulz, M. Baranska, H. H. Belz, P. Rösch, M. A. Strehle, J. Popp, Vib. Spectrosc. 35 (2004) 81 (https://doi.org/10.1016/j.vibspec.2003.12.014)
K. Taraj, I. Malollari, A. Andoni, L. Ciko, P. Lazo, F. Ylli, A. Smeni, A. Como, J. Environ. Prot. Ecol. 18 (2017) 117 (https://scibulcom.net/en/article/Sut8GqESZGR7LPJJlKEw)
M. D. Berechet, E. Manaila, M. D. Stelescu, G. T. Craciun, Rev. Chim. 68 (2017) 2787 (https://doi.org/10.37358/RC.17.12.5979)
Y. Q. Li, D. X. Kong, H. Wu, Ind. Crops Prod. 41 (2013) 269 (https://doi.org/10.1016/j.indcrop.2012.04.056)
A. Andoni, F. Ylli, P. Lazo, K. Taraj, A. Çomo, BSHN (UT) 24 (2017) 152 (https://api.fshn.edu.al/uploads/1_Andoni_et_al_rreg_3bb9aef7ff.pdf)
L. H. de Oliveira, P. Trigueiro, J. S. N. Souza, M. S. de Carvalho, J. A. Osajima, E. C. da Silva-Filho, M. G. Fonseca, Colloids Surfaces, B 209 (2022) 112186 (https://doi.org/10.1016/j.colsurfb.2021.112186)
J. Santos, L. A. Trujillo-Cayado, F. Carrillo, M. L. López-Castejón, M. C. Alfaro-Rod-ríguez, Polymers 14 (2022) 2195 (https://doi.org/10.3390/polym14112195)
X. Nqoro, S. A. Adeyemi, P. Ubanako, D. T. Ndintehal, P. Kumar, Y. E. Choonara, B. A. Aderibigbe, Polym. Bull. 81 (2024) 3459 (https://doi.org/10.1007/s00289-023-04879-2)
M. F. A. Khan, A. Ur Rehman, H. Howari, A. Alhodaib, F. Ullah, Z. ul Mustafa, A. Elaissari, N. Ahmed, Gels 8 (2022) 277 (https://doi.org/10.3390/gels8050277)
Y. Maslii, O. Ruban, G. Kasparaviciene, Z. Kalveniene, A. Materiienko, L. Ivanauskas, A. Mazurkeviciute, D. M. Kopustinskiene, J. Bernatoniene, Molecules 25 (2020) 5018 (https://doi.org/10.3390/molecules25215018)
M. X. Chen, K. S. Alexander, G. Baki, J. Pharm. (Cairo) 2016 (2016) 5754349 (https://doi.org/10.1155/2016/5754349)
J. El Karkouri, M. Bouhrim, O. M. Al Kamaly, H. Mechchate, A. Kchibale, I. Adadi, S. Amine, S. Alaoui Ismaili, T. Zair, Plants 10 (2021) 2068 (https://doi.org/10.3390/plants10102068)
M. G. Martins, D. O. A. Martins, B. L. C. de Carvalho, L. A. Mercante, S. Soriano, M. Andruh, M. D. Vieira, M. G. F. Vaz, J. Solid State Chem. 228 (2015) 99 (https://doi.org/10.1016/j.jssc.2015.04.024)
M. Edraki, D. Zaarei, J. Nanoanalysis 5 (2018) 26 (https://sanad.iau.ir/fa/Journal/jnanoanalysis/DownloadFile/988806)
K. G. Torres, R. R. Almeida, S. Y. de Carvalho, J. F. Haddad, A. A. Leitao, L. G. Guimaraes, Mater. Today Commun. 24 (2020) 101252 (https://doi.org/10.1016/j.mtcomm.2020.101252)
A. Vesković, D. Nakarada, A. Popović Bijelić, Polym. Test. 98 (2021) 107187 (https://doi.org/10.1016/j.polymertesting.2021.107187).