Spectral characterization and antimicrobial activity studies of 5,6-dichloro-1H-benzimidazol-2-yl-(4'/5'/6'-substituted)-phenols (HL1–HL20) and Co(II), Ni(II), Cu(II), Zn(II) and Pd(II) complexes of HL1 Scientific paper
Main Article Content
Abstract
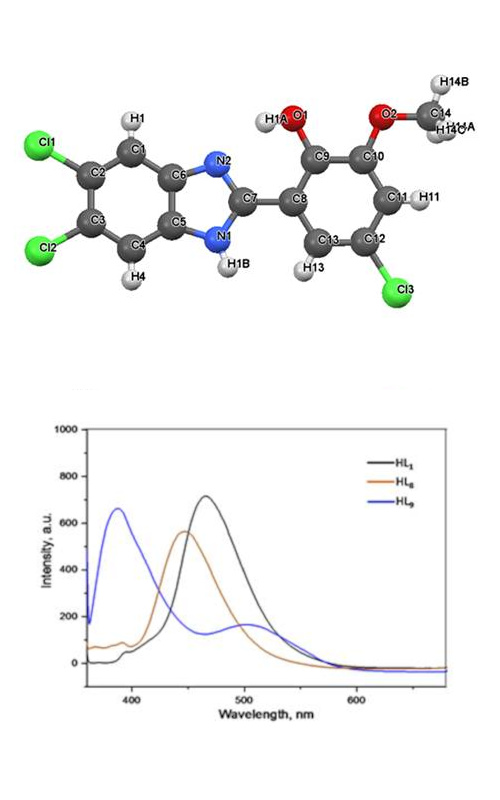
5,6-Dichloro-1H-benzimidazol-2-yl-(4'/5'/6'-substituted)-phenols (HL1–HL20) and MCl2 complexes (M: Co, Ni, Cu, Zn, Pd) of HL1 were synthesized and characterized by various physicochemical and spectroscopic methods such as elemental analysis, thermogravimetric analysis, FTIR, NMR and fluorescence spectroscopy. The structures of the complexes were also confirmed by performing molar conductivity and magnetic moment measurements. HL1 acted as a bidentate, monobasic chelating ligand with NO donor sites in all the complexes. It was found that all complexes have non-electrolytic properties and the M:L ratios are 1:1 in the Zn(II) complex and 1:2 in the other complexes. Crystal structure of HL18 was also investigated. The presence of both intra- and inter-molecular hydrogen bonding was observed in both molecules. According to the fluorescence spectral data, the substituents at the 4-position made the fluorescence emission shifted to the lower wavelengths (redshift) compared to HL1, while the substituents at the 3- and 5-positions caused a blue shift effect. The Zn(II) complex showed a greater redshift effect compared to the other complexes. In addition, antimicrobial activity of the compounds was evaluated against six bacteria and three fungi. It was observed that HL1 and its mono substituted derivatives (HL1–HL11) show selective activity especially against Gram-positive bacteria, Staphylococcus aureus and Staphylococcus epidermidis. Zn(II) complex showed relatively higher activity against Gram-positive bacteria differently from the other complexes.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Istanbul Üniversitesi-Cerrahpasa
Grant numbers 24777
References
M. Rasschaert, D. Schrijvers, J. van den Brande, J. Dyck, J. Bosmans, K. Merkle, J. B. Vermorken, Br. J. Cancer 96 (2007) 1692 (https://doi.org/10.1038/sj.bjc.6603776)
M. Montillo, F. Ricci, A. Tedeschi, E. Vismara, E. Morra, Expert Rev. Hematol. 3 (2010) 131 (https://doi.org/10.1586/ehm.10.7)
C. S. P. Sastry, P. Y. Naidu, S. S. N. Murty, Talanta 44 (1997) 1211 (https://doi.org/10.1016/S0039-9140(97)83117-7)
C. Delescluse, M. P. Piechock, N. Ledirac, R. H. Hines, R. Li, X. Gidrol, R. Rahmani, Biochem. Pharmacol. 61 (2001) 399 (https://doi.org/10.1016/S0006-2952(00)00562-1)
K. K. Mothilal, C. Karunakaran, A. Rajendran, R. Murugesan, J. Inorg. Biochem. 98 (2004) 322 (https://doi.org/10.1016/j.jinorgbio.2003.10.017)
E. J. Cardoso, A. F. Luna, J. P. Urizar, Acta Tropica 92 (2004) 237 (https://doi.org/10.1016/j.actatropica.2004.08.003)
M. Savlik, P. Polaskova, B. Szotakova, J. Lamka, L. Skalova, Res. Vet. Sci. 79 (2005) 139 (https://doi.org/10.1016/j.rvsc.2004.10.007)
R. G. Almeida, J. C. Florio, H. S. Spinosa, M. M. Bernardi, Neurotoxicol. Teratol. 24 (2002) 255 (https://doi.org/10.1016/S0892-0362(02)00203-9)
J. Gronvold, T. S. Svendsen, H. O. Kraglund, J. Bresciani, J. Monrad, Vet. Parasitol. 124 (2004) 91 (https://doi.org/10.1016/j.vetpar.2004.06.003)
B. Pathare, T. Bansode, Results Chem. 3 (2021) 100200 (https://doi.org/10.1016/j.rechem.2021.100200)
S. R. Brishty, M. J. Hossain, M. U. Khandaker, M. R. I. Faruque, H. Osman, S. M. A. Rahman, Front. Pharmacol. 12 (2021) 762807 (https://doi.org/10.3389/fphar.2021.762807)
G. R. Morais, E. Palma, F. Marques, L. Gano, M. C. Oliveira, A. Abrunhosa, H. V. Miranda, A. Paulo, T. F. Outeiro, I. Santos, J. Heterocycl. Chem. 54 (2015) 255 (https://doi.org/10.1002/jhet.2575)
R. Sharma, A. Bali, B. Chandhari, Bioorg. Med. Chem. Lett. 27 (2017) 3007 (https://doi.org/10.1016/j.bmcl.2017.05.017)
A. S. Alpan, S. Parlar, L. Carlino, A. H. Tarikogullari, V. Alptüzün, H. S. Güneş, Bioorg. Med. Chem. 21 (2013) 4928 (https://doi.org/10.1016/j.bmc.2013.06.065)
R. Bonnett, Chem. Rev. 63 (1963) 573 (https://doi.org/10.1021/cr60226a002)
W. Y. Zhu, K. Liu, X. Zhang, Sens. Diagn. 2 (2023) 665 (https://doi.org/10.1039/D3SD00020F)
R. Sathyanarayana, V. Kumar, G. H. Pujar, B. Poojary, M. K. Shankar, S. Yallappa, J. Photochem. Photobiol., A 402 (2020) 112751 (https://doi.org/10.1016/j.jphotochem.2020.112751)
A. Tavman, D. Gürbüz, A. A. Karaçelik, D. N. Çolak, D. Efe, A. Cinarli, Rev. Roum. Chim. 69 (2024) 201 (https://doi.org/10.33224/rrch.2024.69.3-4.10)
A. Tavman, I. Boz, A. S. Birteksöz, Spectrochim. Acta, A 77 (2010) 199 (https://doi.org/10.1016/j.saa.2010.05.008)
A. Tavman, I. Boz, A. S. Birteksöz, A. Cinarli, J. Coord. Chem. 63 (2010) 1398 (https://doi.org/10.1080/00958971003789835)
A. Tavman, D. Gürbüz, S. Oksüz, A. Cinarli, Mor. J. Chem. 6 (2018) 328 (https://doi.org/10.48317/IMIST.PRSM/morjchem-v6i2.7909)
L. Wang, C. X. Zhang, J. Q. Zhao, Chinese J. Struct. Chem. 33 (2014) 1479 (https://caod.oriprobe.com/articles/43884889/Synthesis__Structure_and_Catalytic_Activity_of_a_M.htm)
M. Haghverdi, A. Tadjarodi, N. Bahri-Laleh, M. N. Haghighi, J. Coord. Chem. 71 (2018)1180 (https://doi.org/10.1080/00958972.2018.1446527)
M. Haghverdi, A. Tadjarodi, N. Bahri-Laleh, M. N. Haghighi, Appl. Organomet. Chem. 32 (2018) e4015 (https://doi.org/10.1002/aoc.4015)
T. K. C. Huynh, T. H. A. Nguyen, T. C. T. Nguyen, T. K. D. Hoang, RSC Adv. 10 (2020) 20543 (https://doi.org/10.1039/D0RA02282A)
M. M. Karpinska, J. Matysiak, A. Niewiadomy, Arch. Pharm. Res. 34 (2011) 1639 (https://doi.org/10.1007/s12272-011-1008-0)
H. F. Ridley, G. W. Spickett, G. M. Timmis, J. Het. Chem. 2 (1965) 453 (https://doi.org/10.1002/jhet.5570020424)
Z. Karimi-Jaberi, M. Amir, J. Chem. 9 (2012) 167 (https://doi.org/10.1155/2012/793978)
M27-A3: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard, 3rd ed., Clinical and Laboratory Standards Institute, Wayne, PA, 2012
M100-Ed.31: Performance Standards for Antimicrobial, Clinical and Laboratory Standards Institute, Wayne, PA, 2021
G. M. Sheldrick, Acta Cryst., A 64 (2008) 112 (https://doi.org/10.1107/S0108767307043930)
G. M. Sheldrick, Acta Cryst., C 71 (2015) 3 (https://doi.org/10.1107/S2053229614024218)
APEX2, Bruker AXS Inc., Madison, WI, 2013
C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. van de Streek, P. A. Wood, J. Appl. Cryst. 41 (2008) 466 (https://doi.org/10.1107/S0021889807067908)
L. J. Farrugia, J. Appl. Cryst. 45 (2012) 849 (https://doi.org/10.1107/S0021889812029111)
W. Geary, Coord. Chem. Rev. 7 (1971) 81 (https://doi.org/10.1016/S0010-8545(00)80009-0)
D. Lomjanský, C. Rajnák, J. Titis, J. Monco, L. Smolko, R. Boča, Inorg. Chim. Acta 483 (2018) 352 (https://doi.org/10.1016/j.ica.2018.08.029)
A. Tavman, Spectrochim. Acta, A 63 (2006) 343 (https://doi.org/10.1016/j.saa.2005.05.020)
D. Kanamori, Y. Yamada, A. Onoda, T. A. Okamura, S. Adachi, H. Yamamoto, N. Ueyama, Inorg. Chim. Acta 358 (2005) 331 (https://doi.org/10.1016/j.ica.2004.09.014)
V. M. Leovac, L. S. Jovanović, V. S. Čečljević, L. J. Bjwlica, V. B. Arion, N. V. Gerbelu, Polyhedron 13 (1994) 3005 (https://doi.org/10.1016/S0277-5387(00)83421-X)
N. Sundaraganesan, C. Meganathan, B. Anand, C. Lapouge, Spectrochim. Acta, A 66 (2007) 773 (https://doi.org/10.1016/j.saa.2006.04.025)
D. Mahadevan, S. Periandy, M. Karabacak, S. Ramalingam, Spectrochim. Acta, A 82 (2011) 481 (https://doi.org/10.1016/j.saa.2011.07.082)
P. E. Kazin, M. A. Pogosova, L. A. Trusov, I. V. Kolesnik, O. V. Magdysyuk, R. E. Dinnebier, J. Solid State Chem. 237 (2016) 349 (https://doi.org/10.1016/j.jssc.2016.03.004)
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B, 5th ed., John Wiley & Sons, New York, 1997
A. Tavman, A. Z. Elmal, D. Gürbüz, M. Hacioglu, A. S. Birteksöz Tan, A. Cinarli, Rev. Roum. Chim. 68 (2023) 49 (https://doi.org/10.33224/rrch.2023.68.1-2.05).