A comparative study of ketoprofen-loaded microparticles prepared using emulsion-congealing and solvent evaporation techniques Scientific paper
Main Article Content
Abstract
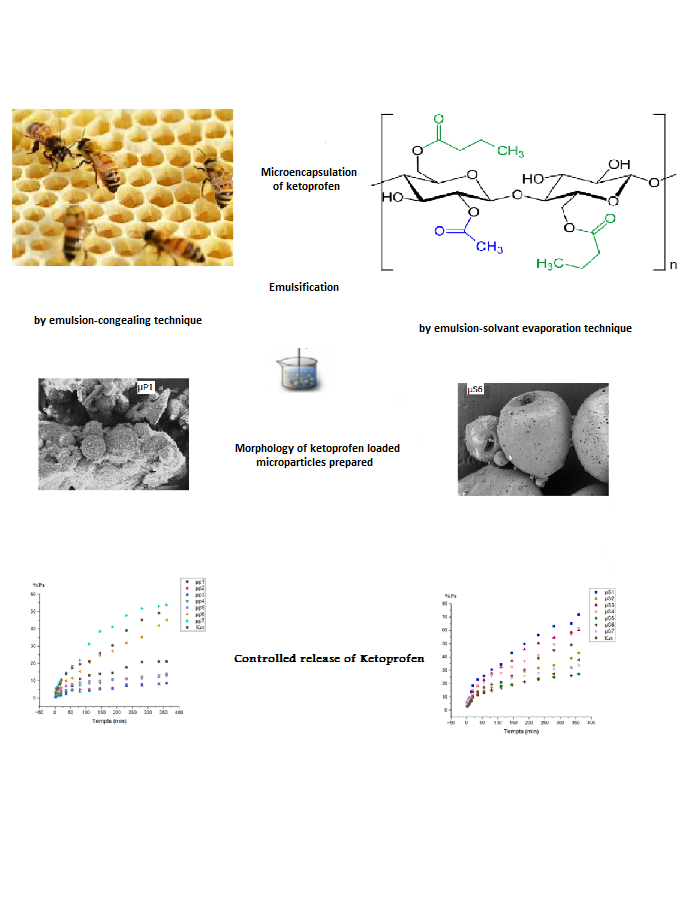
Ketoprofen (Ket) is a commonly used non-steroidal anti-inflammatory drug (NSAID) with analgesic and anti-inflammatory properties. However, its poor aqueous solubility and short biological half-life limit its therapeutic efficacy and patient compliance. Controlled-release microparticles offer a strategy to prolong drug release and improve bioavailability. In this study, we prepared ketoprofen-loaded microparticles using two microencapsulation techniques: emulsion/congealing with beeswax and solvent evaporation with cellulose acetate butyrate (CAB). We then tailored co-matrices containing hydrophobic components (PMMA and PCL) and hydrophilic components (HPMC and β-cyclodextrin) to modulate drug release. Microparticles based on beeswax, particularly when combined with PMMA, exhibited slower release due to reduced matrix permeability. Including hydrophilic excipients in beeswax-based microparticles accelerated the release of ketoprofen by promoting water penetration and drug solubilization. By contrast, the incorporation of hydrophilic excipients into CAB-based microspheres slightly decreased drug release, probably because a denser matrix structure formed during solvent evaporation. These results demonstrate that the encapsulation method and matrix composition both critically influence ketoprofen release kinetics, providing guidance for the rational design of controlled-release drug delivery systems.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
F. Jamali, D. Brocks, Clin. Pharmacokinet. 19 (1990) 197 (https://doi.org/10.2165/00003088-199019030-00004)
D. Wolff, G. Christophersen, C. Brown, S. M. Mulcahey, M. K. Mulcahey, The Phys. Sportsmed. 49 (2021) 381 (https://doi.org/10.1080/00913847.2021.1886573)
R. Rajamohan, E.Kamaraj, P. Muthuraja, K. Murugavel, C. Govindasamy, D. S. Prabakaran, T. Malik, Y. R. Lee, Sci. Rep. 14 (2024) 21516 (https://doi.org/10.1038/s41598-024-71615-9)
A. K. Arifah, M. F. Landoni, S. P. Frean, P. Lees, Am. J. Vet. Res. 62 (2001) 77 (http://dx.doi.org/10.2460/ajvr.2001.62.77)
S. Bindu, S. Mazumder, U. Bandyopadhyay, Biochem. Pharmacol. 180 (2020) 114147 (https://doi.org/10.1016/j.bcp.2020.114147)
K. Sanka, P. R. Veerareddy, R. R. Pragada, J. Hol. Integ. Pharm. 6 (2025) 83 (https://doi.org/10.1016/j.jhip.2025.03.001)
P. R. Sune, K. S. Jumde, P. R. Hatwar, R. L. Bakal, A. V. Korde, GSC Biol. Pharm. Sci. 29 (2024) 286 (https://doi.org/10.30574/gscbps.2024.29.3.0475)
T. K.Giri, C.Choudhary, Ajazuddin, A. Alexander, H. Badwaik, D. K. Tripathi, Saudi Pharm. J. 21 (2013) 125 (http://dx.doi.org/10.1016/j.jsps.2012.05.009)
W. Elballa, M. Salih, A. E. Elawni, J. Phar. Res. Int. 36 (2024) 78 (https://doi.org/10.9734/jpri/2024/v36i127630)
F. Maestrelli, N. Zerrouk, M. Cirri, P. Mura, Int. J. Pharm. 485 (2015) 365 (https://doi.org/10.1016/j.ijpharm.2015.02.073)
R. Bodmeier, H. Chen, J. Contr. Rel. 10 (1989) 167 (https://doi.org/10.1016/0168-3659(89)90059-X)
G. J. Vergote, C. Vervaet, I. V. Driessche, S. Hoste, S. De Smedt, J. Demeester, R. A. Jain, S. Ruddy, J. P. Remon, Int. J. Pharm. 219 (2001) 81 (https://doi.org/10.1016/S0378-5173(01)00628-7)
V. Nahum, A. J. Domb, Int J. Pharm. 621 (2022) 121797 (https://doi.org/10.1016/j.ijpharm.2022.121797)
L. Y. Ho, Z. S. Xiang, R. Gopal, S. A. Khan, Int J. Pharm. 596 (2021) 120230 (https://doi.org/10.1016/j.ijpharm.2021.120230)
M. Üner, U. Gönüllü, G. Yener, T. Altınkurt, Farmaco 60 (2005) 27 (https://doi.org/10.1016/j.farmac.2004.08.008)
M. Ricci, P. Blasi, S. Giovagnoli, C. Rossi, G. Macchiarulo, G. Luca, G. Basta, R. Calafiore, J. Contr. Rel. 107 (2005) 395 (https://doi.org/10.1016/j.jconrel.2005.06.023)
S. Kangishwar, N. Radhika, A. A. Sheik, A. Chavali, S. Hariharan, Polym. Bull. 80 (2023) 47 (https://doi.org/10.1007/s00289-022-04087-4)
F. Ö. Gökmen, N. P. Bayramgil, Carbohydrate Polymers. 297 (2022) 120030 (https://doi.org/10.1016/j.carbpol.2022.120030)
B. Gupta, V. Mishra, S. Gharat, M. Momin, A. Omri, Pharmaceuticals (Basel) 14 (2021) 1201 (https://doi.org/10.3390/ph14111201)
T. Yamada, H. Onishi, Y. Machida, J. Contr. Rel. 75 (2001) 271 (https://doi.org/10.1016/S0168-3659(01)00399-6).
O. C. Larbi, H. Merine, Y. Ramli, F. B. Toumi, K. Guemra, A. Dehbi, J. Serb. Chem. Soc. 83 (2018) 1243 (https://doi.org/10.2298/JSC171112065LA)
K. Badis, H. Merine, Y. Ramli, O. C. Larbi, C. H. Memou, J. Mex. Chem. Soc. 66 (2022) 17 (https://doi.org/10.29356/jmcs.v66i1.1583)
M. Mouffok, A. Mesli, I. Abdelmalek, E. Gontier, J. Serb. Chem. Soc. 81 (2016) 1183 (https://doi.org/10.2298/JSC160308068M)
A. Merdoud, M. Mouffok, A. Mesli, N. Chafi, M. Chaib, J. Serb. Chem. Soc. 85 (2020) 531 (https://doi.org/10.2298/JSC190326132M)
R. Brahmi, K. Diaf, Z. ELBahri, M. Baitiche, J. Serb. Chem. Soc. 89 (2024) 91 (https://doi.org/10.2298/JSC230501088B)
D. J. Hines, D. L. Kaplan, Crit. Rev. Ther. Drug Carrier Syst. 30 (2013) 257 (https://doi.org/10.1615/critrevtherdrugcarriersyst.2013006475)
O. Khoukhi, Z. El Bahri, K. Diaf, M. Baitiche, Chem. Pap. 70 (2016) 0014 (https://doi.org/10.1515/chempap-2016-0014).
E. Obaid, A. K. M. Jamil, S. Prabu, S. M. Saharin, S. Mohamad, Spectrochim. Acta, A 241 (2020) 118674 (https://doi.org/10.1016/j.saa.2020.118674).
G. Gupta, K. Anjali, IOP Conf. Ser.: Earth Environ. Sci. 1110 (2023) 012041 (https://doi.org/10.1088/1755-1315/1110/1/012041)
G. Champetier, L. Monnerie, Introduction à la Chimie Macromoléculaire, Hermann, Paris, 1975
J. Szejtli, Cyclodextrin Technology, Kluwer Academic Publishers, Dordrecht, 1988, pp. 1–78
V. J. Stella, M. R. Venkatramana, E. A. Zannou, V. Zia, Adv. Drug Deliv. Rev. 36 (1999) 3 (https://doi.org/10.1016/S0169-409X(98)00052-0)
G. Poovi, S. Rajpriyadarsini, S. Uma, R. Vinothini, Asian J. Pharm. Sci. 10 (2015) 433 (https://doi.org/10.1016/j.ajps.2015.05.001)
L. Battaglia, M. Gallarate, P. P. Panciani, E. Ugazio, S. Sapino, E. Peira, D. Chirio, in Application of Nanotechnology in Drug Delivery, A. D. Sezer, Ed., InTechOpen LTD, London, 2014 (http://dx.doi.org/10.5772/58405)
R. H. Müller, K. Mader, S. Gohla, Eur. J. Pharm. Biopharm. 50 (2000) 161 (https://doi.org/10.1016/S0939-6411(00)00087-4)
R. Dinarvand, S. H. Moghadam, A. Sheikhi, F. Atyabi, J. Microencapsul. 22 (2005) 139 (https://doi.org/10.1080/02652040400026392)
A. Müllertz, A. Ogbonna, S. Ren, T. Rades, J. Pharm. Pharmacol. 62 (2010) 1622 (http://dx.doi.org/10.1111/j.2042-7158.2010.01107.x)
R. B. Pedada, E. Vanka, A. M. S. Sudhakar Babu, P. K. Desu, P. R. Bharathi, P. V. Rao, PharmaTutor 1 (2013) 60 (http://www.pharmatutor.org/pdf_download/pdf/1(2)-enhancement-of-solubility-an-over-view.pdf)
A. K. Hassan, Indian J. Pharm. Sci. 80 (2018) 334 (https://doi.org/10.36468/pharmaceutical-sciences.561)
E. Kim, W. G. Cho, J. Korean Appl. Sci. Technol. 31 (2014) 203 (http://dx.doi.org/10.12925/jkocs.2014.31.2.203).