Green and efficient synthesis of new β-amido-aroyl carbonyl derivatives catalyzed by choline chloride/urea as a deep eutectic solvent Scientific paper
Main Article Content
Abstract
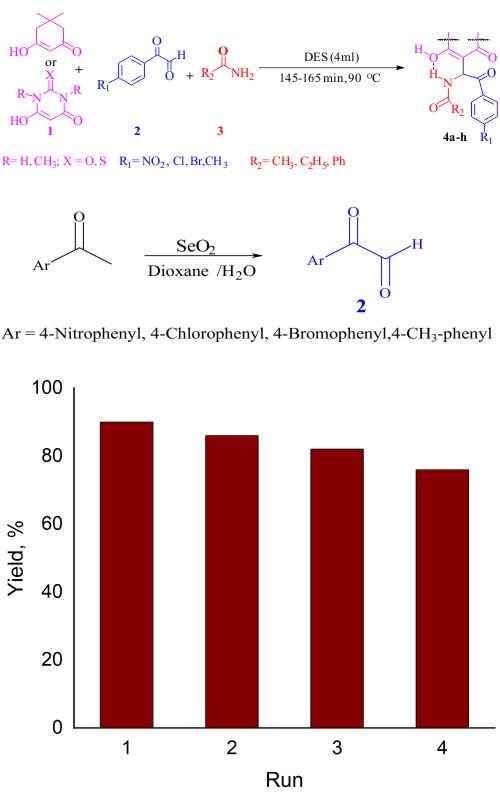
A green and highly efficient synthesis of some new β-amido-aroyl carbonyl derivatives has been achieved through a one-pot, three-component reaction of dimedone/barbituric acid derivatives, arylglyoxals, and amides in choline chloride/urea as a deep eutectic solvent (DES). The use of biodegradable materials, short reaction time and high yields of products introduced this protocol as an efficient environmentally friendly method. The DES could be easily recovered and reused about four times with satisfied catalytic activity.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
B. M. Trost, Science 254 (1991) 1471 (https://doi.org/10.1126/science.1962206)
S. Pal, M. Nasim Khan, S. Karamthulla , L. H. Choudhury, Tetrahedron Lett. 56 (2015) 359 (https://doi.org/10.1016/j.tetlet.2014.11.095)
A. J. Von Wangelin, H. Neumann, D. Gördes, S. Klaus, D. Strübing , M. Beller, Chem. Eur. J. 9 (2003) 4286 (https://doi.10.1002/chem.200305048)
P. T. Anastas, Crit. Rev. Anal. Chem. 29 (1999) 167 (https://doi.org/10.1080/10408349891199356)
P. Liu, J. W. Hao, L. P. Mo, Z. H. Zhang. RSC Adv. 5 (2015) 48675 (https://doi.org/10.1039/c5ra05746a)
Y. Cui, C. Li, J. Yin, S. Li, Y. Jia, M. Bao. J. Mol. Liq. 236 (2017) 338 (https://doi.org/10.1016/i.gee.2019.03.002)
Q. Zhang, K. De.Oliveira Vigier, S. Royer, F. Jerome, Chem. Soc. Rev. 41 (2012) 7108 ( https://doi.org/10.1039/c2cs35178a)
N. Azizi, T. Soleymani Ahooi , M. Mahmoudi Hashemi, J. Mol. Liq. 246 (2017 ) 221 (https://doi.org/10.1016/j.molliq.2017.09.049)
P. Liu, J.-W. Hao, L.-P. Mo, Z.-H. Zhang. RSC Adv. 5 (2015) 48675 (https://doi.org/10.1039/c5ra05746a)
E. Habibi, K. Ghanemi, M. Fallah-Mehrjardi, A. Dadolahi-Sohrab, Anal. Chim. Acta 762 (2013) 61 (https://doi.10.1016/j.aca.2012.11.054)
A. Shaabani, S. E. Hooshmand, A. Tavousi Tabatabaei, Tetrahedron Lett. 57 (2016) 351 (https://doi.org/10.1016/j.tetlet.2015.12.017)
A. K. Sanap, G. S. Shankarling, RSC Adv. 4 (2014) 34938 (https://doi.org/10.1039/C4RA05858E)
A. P. Abbott, R. C. Harris, K. S. Ryder, C. DAgostino, L. F. Gladden, M. D. Mantle, Green Chem. 13 (2011 ) 82 (https://doi.org/10.1039/C0GC00395F)
S. Khandelwal, Y. K. Tailor, M. Kumar, J. Mol. Liq. 215 (2016) 345 (https://doi.org/10.1016/j.molliq.2015.12.015)
E. L. Smith, A. P. Abbott, K. S. Ryder, Chem. Rev. 114 (2014) 11060 (https://doi.org/10.1021/cr.300162p)
J. Barluenga, B. Olano, S. Fustero, J. Org. Chem. 50 (1985) 4052 (https://doi.org/10.1021/jo00221a018)
I. Nageshwar Rao, E. N. Prabhakaran, S. Kumar Das, J. Iqbal, J. Org. Chem. 68 (2003) 4079 (https://doi.org/10.1021/jo020559c)
R. P. Cheng, S. H. Gellman, W. F. De. Grado, Chem. Rev. 101 (2001) 3219. (https://doi.org/10.1021/cr000045i)
U. Dahn, H. Hagenmaier, H. Hohne, W. A. König, G. Wolf, H. Zahner. Arch. Microbiol. 107 (1997) 143 (https://doi.org/10.1007/bf00446834)
R. M. Kumbhare, M. Sridhar, J. Chem. Sci. 124 (2012) 495 (https://doi.org/10.1007/s12039-011-0183-3)
G. L. Buchanan, Chem. Soc. Rev. 17 (1988) 91 (https://doi.org/10.1039/CS9881700091)
D. Bahulayan, S. K. Das, J. Iqbal, J. Org. Chem. 68 (2003) 5735 (https://doi.org/10.1021/jo02734p)
A. Javid, M. M. Heravi , F. F. Bamoharram, Monatsh. Chem. 143 (2012) 831 (https://doi.org/10.1007/s00706-011-0669-1)
24. R. Khoeiniha, A. Olyaei, M. Saraei, J. Heterocyclic Chem. 54 (2017) 1746 (https://doi.org/10.1002/jhet.2752)
O. N. Petrova1, L. L. Zamigajlo, K. S. Ostras, S. V. Shishkina, O. V. Shishkin, A. V. Borisov, V. I. Musatov1, M. G. Shirobokova, V. V. Lipson, Chem. Heterocycl. Comp. 51 (2015) 310 (https://doi.org/10.1007/s10593-015-1700-y)
F. Jafari, S. Kodabakhshi , S. Gharehzadeh Shirazi, RSC Adv. 4 (2014) 48095 (https://doi.org/10.1039/C4RA90049A)
S. Khodabakhshi, B. Karami, Tetrahedron Lett. 55 (2014) 7136 (https://doi.org/10.1016/j.tetlet.2014.11.016)
S. Khodabakhshi, M. Khaleghi Abbasabadi, M. Baghrnejad, F. Marahel, J. Chin. Chem. Soc. 62 (2015) 9 (https://doi.org/10.1002/jccs.201400266)
B. Khalili, P. Jajarmi, B. Eftekhari-Sis , M. M. Hashemi, J. Org. Chem. 73 (2008) 2090 (https:// doi.org/10.1021/jo702385n)
J. Khalafy, M. Ezzati, M. Rimaz, A.d Poursattar Marjani, H. Yaghoobnejad Asl, J. Iran. Chem. Soc. 11 (2014) 1067 (https://doi.org/10.1007/s13738-013-0378-2)
J. Khalafy, M. Rimaz, M. Ezzati, R. H. Prager, Bull. Korean Chem. Soc. 33 (2012) 9 (http://dx.doi.org/10.5012/bkcs.2012.33.9.2890)