Physicochemical characterisation of dihydro-alpha-lipoic acid interaction with human serum albumin by multi-spectroscopic and molecular modelling approach Scientific paper
Main Article Content
Abstract
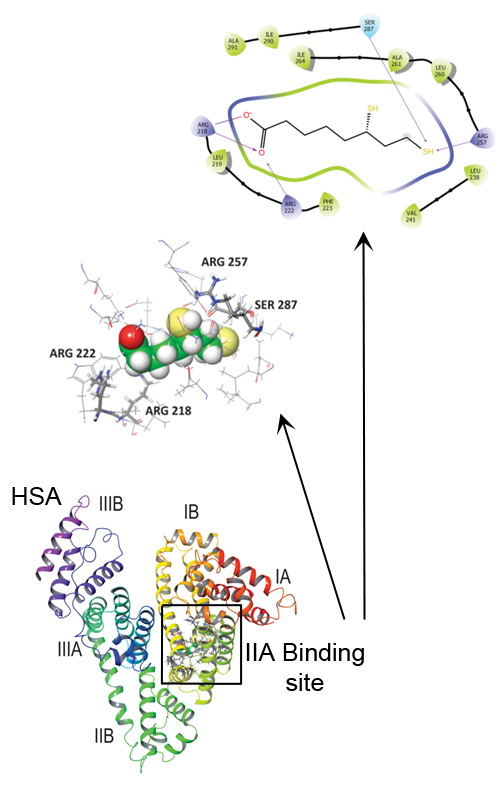
The binding of a popular food supplement and well-known antioxidant, dihydro-alpha-lipoic acid (DHLA) to human serum albumin (HSA) was characterised. The binding was monitored by several spectroscopic methods together with the molecular docking approach. HSA was able to bind DHLA with moderate affinity, 1.00±0.05×104 M-1. Spectroscopic data demonstrated that the preferential binding site for DHLA on HSA is IIA (Sudlow I). Both experimental and molecular docking analysis identified electrostatic (salt bridges) and hydrogen bonds as the key interactions involved in DHLA binding to HSA. Molecular docking confirmed that the Sudlow I site could accommodate DHLA and that the ligand is bound to the protein in a specific conformation. The molecular dynamic simulation showed that the formed complex is stable. Binding of DHLA does not affect the structure of the protein, but it thermally stabilises HSA. Bound DHLA had no effect on the susceptibility of HSA to trypsin digestion. Since DHLA is a commonly used food supplement, knowledge of its pharmacokinetics and pharmacodynamic properties in an organism is very important. This study further expands it by providing a detailed analysis of its interaction with HSA, the primary drug transporter in the circulation.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
R. E. Wang, L. Tian, Y.-H. Chang, J. Pharm. Biomed. Anal. 63 (2012) 165 (https://doi.org/10.1016/j.jpba.2011.12.035)
A. D. McLachlan, J. E. Walker, J. Mol. Biol. 112 (1977) 543 (https://doi.org/10.1016/S0022-2836(77)80163-0)
P. Lee, X. Wu, Curr. Pharm. Des. 21 (2015) 1862 (https://doi.org/10.2174/1381612821666150302115025)
I. D. Pavićević, V. B. Jovanović, M. M. Takić, A. Z. Penezić, J. M. Aćimović, L. M. Mandić, Chem. Biol. Interact. 224 (2014) 42 (https://doi.org/10.1016/j.cbi.2014.10.008)
M. Fasano, S. Curry, E. Terreno, M. Galliano, G. Fanali, P. Narciso, S. Notari, P. Ascenzi, IUBMB Life 57 (2005) 787 (https://doi.org/10.1080/15216540500404093)
R. I. Horowitz, P. R. Freeman, Med. Hypotheses J. 143 (2020) 109851 (https://doi.org/10.1016/j.mehy.2020.109851)
C. Zuliani, L. Baroni, Antioxidants for the Prevention and Treatment of Multiple Sclerosis: An Overview, in Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease, R. R. Watson, V. R. Preedy, Ed(s)., Elsevier Inc., Amsterdam, Netherlands , 2015, p. 341 (https://doi.org/10.1016/B978-0-12-411462-3.00035-7)
S. Salinthone, V. Yadav, D. N. Bourdette, D. W. Carr, Endocrine Metab. Immune Disord. Targets. 8 (2008) 132 (https://doi.org/10.2174/187153008784534303)
A. R. Smith, S. V. Shenvi, M. Widlansky, J. H. Suh, T. M. Hagen, Curr. Med. Chem. 11 (2004) 1135 (https://doi.org/10.2174/0929867043365387)
T. Kawabata, L. Packer, Biochem. Biophys. Res. Commun. 203 (1994) 99 (https://doi.org/10.1006/bbrc.1994.2154)
G. Suji, S. A. Khedkar, S. K. Singh, N. Kishore, E. C. Coutinho, V. M. Bhor, S. Sivakami, Protein J. 27 (2008) 205 (https://doi.org/10.1007/s10930-008-9126-3)
P. Atukeren, S. Aydin, E. Uslu, M. K. Gumustas, U. Cakatay, Oxid. Med. Cell. Longev. 3 (2010) 206 (https://doi.org/10.4161/oxim.3.3.11786)
N. Perricone, K. Nagy, F. Horváth, G. Dajkó, I. Uray, I. Zs.-Nagy, Arch. Gerontol. Geriatr. 29 (1999) 45 (https://doi.org/10.1016/S0167-4943(99)00022-9)
J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer US, New York, USA, 2006 (https://doi.org/10.1007/978-0-387-46312-4)
J. M. Vasquez, A. Vu, J. S. Schultz, V. I. Vullev, Biotechnol. Prog. 25 (2009) 906 (https://doi.org/10.1002/btpr.188)
S. K. Chaturvedi, M.K. Siddiqi, P. Alam, M. Zaman, R.K. Khan, RSC Adv. 6 (2016) 85860 (https://doi.org/10.1039/C6RA10487H)
N. Zaidi, E. Ahmad, M. Rehan, G. Rabbani, M. R. Ajmal, Y. Zaidi, N. Subbarao, R. K. Khan, J. Phys. Chem. B 117 (2013) 2595 (https://doi.org/10.1021/jp3069877)
P. D. Ross, S. Subramanian, Biochemistry 20 (1981) 3096 (https://doi.org/10.1021/bi00514a017)
A. A. Salem, M. Lotfy, A. Amin, M. A. Ghattas, Biochem. Biophys. Reports. 20 (2019) 100670 (https://doi.org/10.1016/j.bbrep.2019.100670)
Q. Li, W. Yang, L. Qu, H.-Y. Qi, Y. Huang, Z. Zhang, J. Spectrosc. 2014 (2014) Article ID 834501 (https://doi.org/10.1155/2014/386586)
A. Ploch-Jankowska, D. Pentak, Pharmaceuticals 13 (2020) 205 (https://doi.org/10.3390/ph13090205)
L. Liang, H.A. Tajmir-Riahi, M. Subirade, Biomacromolecules. 9 (2008) 50 (https://doi.org/10.1021/bm700728k)
S. L. Minic, M. Milcic, D. Stanic-Vucinic, M. Radibratovic, T.G. Sotiroudis, M.R. Nikolic, T.Ć. Velickovic, RSC Adv. 5 (2015) 61787 (https://doi.org/10.1039/c5ra05534b)
N. Gligorijević, V. Šukalović, A. Penezić, O. Nedić, Int. J. Biol. Macromol. 147 (2020) 319 (https://doi.org/10.1016/j.ijbiomac.2020.01.098)
M. Radibratovic, S. Minic, D. Stanic-Vucinic, M. Nikolic, M. Milcic, T.C. Velickovic, PLoS One. 11 (2016) e0167973 (https://doi.org/10.1371/journal.pone.0167973)
B.E. Lang, K.D. Cole, Biotechnol. Prog. 31 (2015) 62 (https://doi.org/10.1002/btpr.1996)
D.P. Yeggoni, A. Rachamallu, R. Subramanyam, J. Photochem. Photobiol. B Biol. 160 (2016) 248 (https://doi.org/10.1016/j.jphotobiol.2016.04.012)
T. Sjödin, R. Hansson, I. Sjöholm, Biochim. Biophys. Acta. 494 (1977) 61 (https://doi.org/10.1016/0005-2795(77)90135-0)
A. Kawakami, K. Kubota, N. Yamada, U. Tagami, K. Takehana, I. Sonaka, E. Suzuki, K. Hirayama, FEBS J. 273 (2006) 3346 (https://doi.org/10.1111/j.1742-4658.2006.05341.x)
K. Oettl, R.E. Stauber, Br. J. Pharmacol. 151 (2007) 580 (https://doi.org/10.1038/sj.bjp.0707251)
I. Sadowska-Bartosz, S. Galiniak, G. Bartosz, Molecules. 19 (2014) 4880 (https://doi.org/10.3390/molecules19044880)