Antimicrobial and anticancer activities of copolymers of tri-O-acetyl-D-glucal and itaconic anhydride Scientific paper
Main Article Content
Abstract
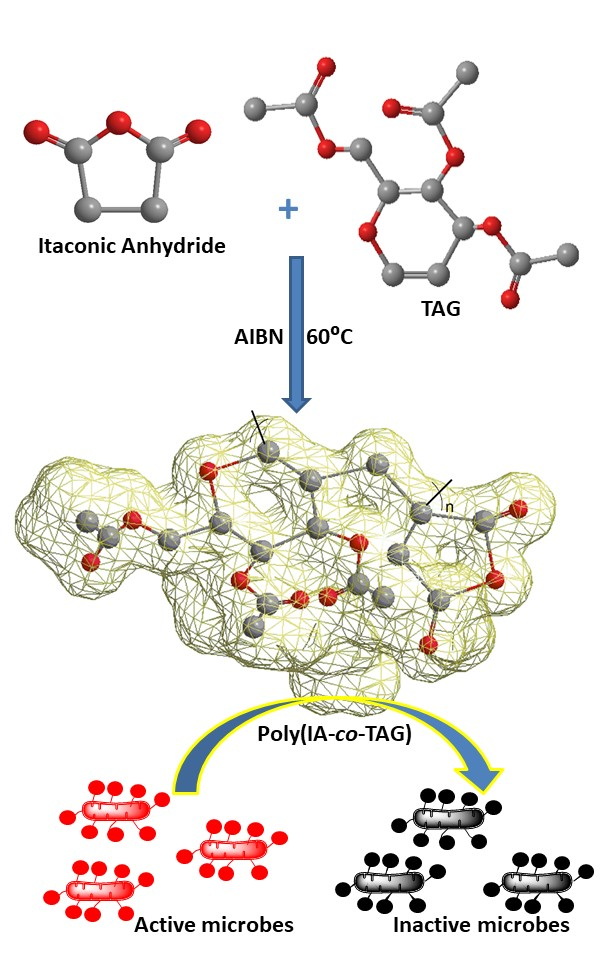
This paper reports the synthesis and characterization of monomers itaconic anhydride (IA) and tri-O-acetyl-d-glucal (TAG) as well as 4,6-di-O-acetyl-d-glucal (PSG). The homopolymers and copolymers of IA and TAG were synthesized via free radical copolymerization in bulk, using azobisisobutyronitrile as an initiator with different feed ratios of monomers. Their structural, molecular and thermal characterization was done using 1H-NMR spectroscopy, gel permeation chromatography and differential scanning calorimetry, respectively. The glass transition temperature (Tg) of copolymers was found in the range of 139–145 °C. The highest Tg was found for IA–TAG2 copolymers, whereas IA–TAG4 copolymer showed lowest Tg. The molecular weight of the copolymers was in the range 5157–5499 g mol-1. The monomer TAG undergoes Ferrier rearrangement in water to give PSG. The antimicrobial activity of IA, TAG, PSG and IA–TAG copolymers was studied using the minimum microbicidal concentration-broth dilution method. TAG, IA and PSG, as well as homopolymer and copolymers of IA and TAG are excellent antimicrobial agents.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
C. Cesa-Luna, A. Baez, V. Quintero-Hernández, J. De La Cruz-Enríquez, M. D. Castañeda-Antonio, J. Muñoz-Rojas, Acta Biol. Colomb. 25 (2020) 140 (http://dx.doi.org/10.15446/abc.v25n1.76867)
P. K. Mantravadi, K. A. Kalesh, R. C. J. Dobson, A. O. Hudson, A. Parthasarathy, Antibiotics 8 (2019) 8 (http://doi.org/10.3390/antibiotics8010008)
P. K. Senthilkumar, D. Reetha, Eur. Rev. Med. Pharmacol. Sci. 15 (2011) 1034 (http://www.europeanreview.org/article/1031)
C. M. Franco, B. I. Vázquez, Antibiotics 9 (2020) 217 (http://dx.doi.org/10.3390/antibiotics9050217)
E. Chiellini, F. Chiellini, P. Cinelli, in Degradable Polymers, G. Scott, Ed., Springer, Dordrecht, 2002, p. 163 (http://doi.org/10.1007/978-94-017-1217-0_7)
Y. Zhu, C. Romain, C. K. Williams, Nature 540 (2016) 354 (http://dx.doi.org/10.1038/nature21001)
G. L. Gregory, E. M. Lopez-Vidal, A. Buchard, Chem. Commun. 53 (2017) 2198 (http://dx.doi.org/10.1039/c6cc09578j)
W. Priebe, I. Fokt, G. Grynkiewicz, in Glycoscience, B. Fraser-Reid, K. Tatsuta, J. Thiem, Eds., Springer-Verlag, Berlin, 2008, p. 699 (http://dx.doi.org/10.1007/978-3-540-30429-6_15)
T. Willke, K.-D. Vorlop, Appl. Microbiol. Biotechnol. 56 (2001) 289 (http://dx.doi.org/10.1007/s002530100685)
S. Shang, S. J. Huang, R. A. Weiss, Polymer 50 (2009) 3119 (http://dx.doi.org/10.1016/j.polymer.2009.05.012)
R. Chauhan, V. Choudhary, J. Appl. Polym. Sci. 109 (2008) 987 (http://doi.org/10.1002/app.28099)
L. Sollka, K. Lienkamp, Macromol. Rapid Commun. 42 (2021) 2000546 (http://dx.doi.org/10.1002/marc.202000546)
S. M. Osman, M. H. El-Newehy, S. S. Al-Deyab, A. El-Faham, Chem. Cent. J. 6 (2012) (http://dx.doi.org/10.1186/1752-153X-6-85)
P. S. Nayak, B. Narayana, B. K. Sarojini, S. Sheik, K. S. Shashidhara, K. R. Chandrashekar, J. Taibah Univ. Sci. 10 (2016) 823 (http://doi.org/10.1016/j.jtusci.2014.09.005)
R. Ashique, R. Chirakal, G. J. Schrobilgen, D. W. Hughes, T. Farncombe, K. Gulenchyn, R. Labiris, T. Truman, C. Saab, in ACS Symp. Ser. A, Vol. 1003, A. Gakh, K. L. Kirk, Eds., American Chemical Society, Washington DC, , 2009, pp. 211–235 (http://dx.doi.org/10.1021/bk-2009-1003.ch010)
M.-J. Han, C.-W. Lee, K.-H. Kim, W.-Y. Lee, Bull. Korean Chem. Soc. 12 (1991) 85 (http://www.koreascience.or.kr/article/JAKO199113464455707.page)
C. Cottet, A. G. Salvay, M. A. Peltzer, M. Fernández-García, Polymers 13 (2021) 200 (https://doi.org/10.3390/polym13020200)
R. Chauhan, V. Choudhary, J. Appl. Polym. Sci. 115 (2010) 491 (https://doi.org/10.1002/app.30824)
K. Bae, S. H. Koo, W. Seo, Arch. Pharm. Res. 14 (1991) 41 (https://doi.org/10.1007/BF02857812)
Y. Xie, W. Yang, F. Tang F., X. Chen, L. Ren, Curr. Med. Chem. 22 (2015) 132 (https://doi.org/10.2174/0929867321666140916113443)
F. Farhadi, B. Khameneh, M. Iranshahi, M. Iranshahy, Phytother. Res. 33 (2019) 13 (https://doi.org/10.1002/ptr.6208)
T. J. Cuthbert, B. Hisey, T. D. Harrison, J. F. Trant, E. R. Gillies, P. J. Ragogna, Angewandte Chemie 57 (2018) 12707 (https://doi.org/10.1002/anie.201806412)
G. L. Y. Woo, M. W. Mittelman, J. P. Santerre Biomaterials 21 (2000) 1235 (https://doi.org/10.1016/s0142-9612(00)00003-x).