Unveiling the regioselective synthesis of antiviral 5-isoxazol-5-yl-2'-deoxyuridines from the perspective of a molecular electron density theory Scientific paper
Main Article Content
Abstract
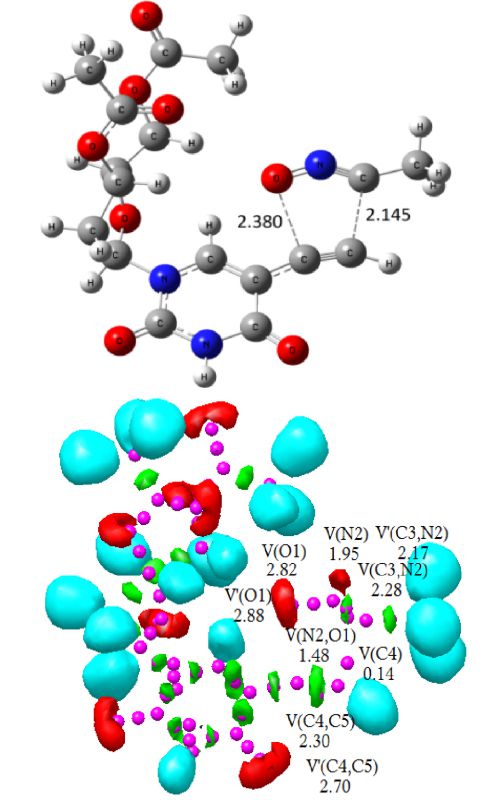
The regioselective synthesis of a potent antiviral sugar nucleoside isoxazole analogue in the [3+2] cycloaddition (32CA) reaction of acetonitrile-N-oxide (ANO) and acetyl-protected 5-ethynyl-2’-deoxyuridine (EDU) has been studied at the MPWB1K/6-311G(d,p) level within perspective of the molecular electron density theory (MEDT). From an electron localization function (ELF) analysis, ANO is classified as a zwitterionic species devoid of any pseudoradical or carbenoid centre. The ortho regioisomer is energetically preferred over the meta one by the activation enthalpy of 21.7–24.3 kJ mol-1, suggesting complete regioselectivity in agreement with the experiment. The activation enthalpy increases from 53.9 kJ mol-1 in the gas phase to 71.5 kJ mol-1 in water, suggesting more facile reaction in low polar solvents. The minimal global electron density transfer (GEDT) at the TSs suggests non-polar character and the formation of new covalent bonds has not been started at the located TSs, showing non-covalent intermolecular interactions from an atoms-in-molecules (AIM) study and in the independent gradient model (IGM) isosurfaces. The AIM analysis shows more accumulation of electron density at the C–C interacting region relative to the C–O one, and earlier C–C bond formation is predicted from a bonding evolution theory (BET) study.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
J. Jampilek, Molecules 24 (2019) 3839 (https://doi.org/10.3390/molecules24213839)
A. P. Taylor, R. P. Robinson, Y. M. Fobian, D. C. Blakemore, L. H. Jones, O. Fadeyi, Org. Biomol. Chem. 14 (2016) 6611 (https://doi.org/10.1039/C6OB00936K)
A. Padwa, W. H. Pearson, Synthetic Application of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products, Wiley, New York, 2002 (https://doi.org/10.1002/0471221902)
Y. Walunj, P. Mhaske, P. Kulkarni, Mini-Rev. Org. Chem. 18 (2021) 55 (https://doi.org/10.2174/1570193X17999200511131621)
E. Rajanarendar, S. Rama Krishna, D. Nagaraju, K. G. Reddy, B. Kishore, Y. N. Reddy, Bioorg. Med. Chem. Lett. 25 (2015) 1630 (https://doi.org/10.1016/j.bmcl.2015.01.041)
P. Vitale, MG Perrone, P. Malerba, A. Lavecchia, A. Scilimati, Eur. J. Med. Chem. 74 (2014) 606 (https://doi.org/10.1016/j.ejmech.2013.12.023)
R. J. Rama Rao, A. K. S. B. Rao, N. Sreenivas, B. S. Kumar, Y. L. N. Murthy, J. Korean. Chem. Soc. 55 (2011) 243 (https://doi.org/10.5012/jkcs.2011.55.2.243)
L-F. Yu, W. Tückmantel, J. B. Eaton, B. Caldarone, A. Fedolak, T. Hanania, D. Brunner, R. J. Lukas, A. P. Kozikowski, J. Med. Chem. 55 (2012) 812 (https://doi.org/10.1021/jm201301h)
B. Frølund, L. S. Jensen, S. I. Storustovu, T. B. Stensbøl, B. Ebert, J. Kehler, P. Krogsgaard-Larsen, T. Liljefors, J. Med. Chem. 50 (2007) 1988 (https://doi.org/10.1021/jm070038n)
N. Agarwal, P. Mishra, Med. Chem. Res. 27 (2018) 1309 (https://doi.org/10.1007/s00044-018-2152-6)
J. Zhu, J. Mo, H-z. Lin, Y. Chen, Hao-peng Sun, Bio. Med. Chem. 26 (2018) 3065 (https://doi.org/10.1016/j.bmc.2018.05.013)
A. M. Eid ,M. Hawash,J. Amer, A. Jarrar, S. Qadri, I. Alnimer,A. Sharaf, R. Zalmoot, O. Hammoudie,S. Hameedi, A. Mousa, BioMed Res. Int. (2021) 6633297(https://doi.org/10.1155/2021/6633297)
L. Claisen, Ber der Dtsch Chem Ges. 36 (1903) 3664 (https://doi.org/10.1002/cber.190303603168)
T. V. Hansen, P. Wu, V. V. Fokin, J. Org. Chem. 70 (2005) 7761 (https://doi.org/10.1021/jo050163b)
L.-E. Carloni, S. Mohnani,D. Bonifazi, Eur. J. Org. Chem. (2019) 7322(https://doi.org/10.1002/ejoc.201901045)
K. L. Seley-Radtke, M. K. Yates, Antivir. Res. 154 (2018) 66 (https://doi.org/10.1016/j.antiviral.2018.04.004)
L. P. Jordheim, D. Durantel, F. Zoulim, C. Dumontet, Nat. Rev. Drug. Discov. 12 (2013) 447 (https://doi.org/10.1038/nrd4010)
E. Ichikawa, K. Kato, Curr. Med. Chem. 8 (2001) 385 (https://doi.org/10.2174/0929867013373471)
Y-S. Lee, S. M. Park, B. H. Kim, Bioorg. Med. Chem. Lett. 19 (2009) 1126 (https://doi.org/10.1016/j.bmcl.2008.12.103)
L. R. Domingo, Molecules 21 (2016) 1319 (https://doi.org/10.3390/molecules21101319)
L. R. Domingo, N. Acharjee, Molecular Electron Density Theory: A New Theoretical Outlook on Organic Chemistry. in Frontiers in Computational Chemistry, Z. Ul-Haq, A. K. Wilson, Eds., Bentham and Science, Singapore, 2020, pp. 174–227 (https://doi.org/10.2174/9789811457791120050007)
L. R. Domingo, N. Acharjee, New J. Chem. 44 (2020) 13633 (https://doi.org/10.1039/D0NJ02711A)
L. R. Domingo, M. R. Gutiérrez, N. Acharjee, Chemistry 3 (2021) 74 (https://doi.org/10.3390/chemistry3010006)
A. D. Becke, K. E. Edgecombe, J. Chem. Phys. 92 (1990) 5397 (https://doi.org/10.1063/1.458517)
B. Silvi, A. Savin, Nature 371 (1994) 683 (https://www.nature.com/articles/371683a0)
R. G. Parr, W. Yang, Density functional theory of atoms and molecules, Oxford University Press, New York, 1989
L. R. Domingo, M. R. Gutiérrez, P. Pérez, Molecules 21 (2016) 748 (https://doi.org/10.3390/molecules21060748)
S. J. Moss, C. J. Coady, J. Chem. Educ. 60 (1983) 455 (https://doi.org/10.1021/ed060p455)
L. R. Domingo, RSC Adv. 4 (2014) 32415 (https://doi.org/10.1039/C4RA04280H)
R. F. W. Bader, In Atoms in Molecules: A Quantum Theory, Clarendon Press, New York, 1990
R. F. W. Bader, H. Essén, J. Chem. Phys. 80 (1984) 1943 (https://doi.org/10.1063/1.446956)
C. Lefebvre, H. Khartabil, J.-C. Boisson, J. Contreras‐García, J.-P. Piquemal, E. Hénon, ChemPhysChem 19 (2018) 724 (https://doi.org/10.1002/cphc.201701325)
F. De Proft, R. V-Reyes, A. Peeters, C. Von Alsenoy, P. Geerlings, J. Comput. Chem. 24 (2003) 463 (https://doi.org/10.1002/jcc.10241)
X. Krokidis, S. Noury, B. Silvi, J. Phys. Chem., A 101 (1997) 7277. (https://doi.org/10.1021/jp9711508)
L. R. Domingo, M. J. Aurell, P. Pérez, R. Contreras, Tetrahedron 58 (2002) 4417 (https://doi.org/10.1016/S0040-4020(02)00410-6)
L. R. Domingo, P. Pérez, Org. Biomol. Chem. 9 (2011) 7168 (https://doi.org/10.1039/C1OB05856H)
R. G. Parr, R. G. Pearson, J. Am. Chem. Soc. 105 (1983) 7512 (https://doi.org/10.1021/ja00364a005)
R. G. Parr, L. von Szentpaly, S. Liu, J. Am. Chem. Soc. 121 (1999)1922 (https://doi.org/10.1021/ja983494x)
L. R. Domingo, M. R. Gutiérrez, P. Pérez, RSC Adv. 10 (2020) 15394 (https://doi.org/10.1039/D0RA01548B)
R. Thom, Stabilité Structurelle et Morphogenèse, Interéditions, Paris, 1972 (ISBN 2-7296-0081-7).