Copper ions biosorption onto bean shells: Kinetics, equilibrium and process optimization studies Scientific paper
Main Article Content
Abstract
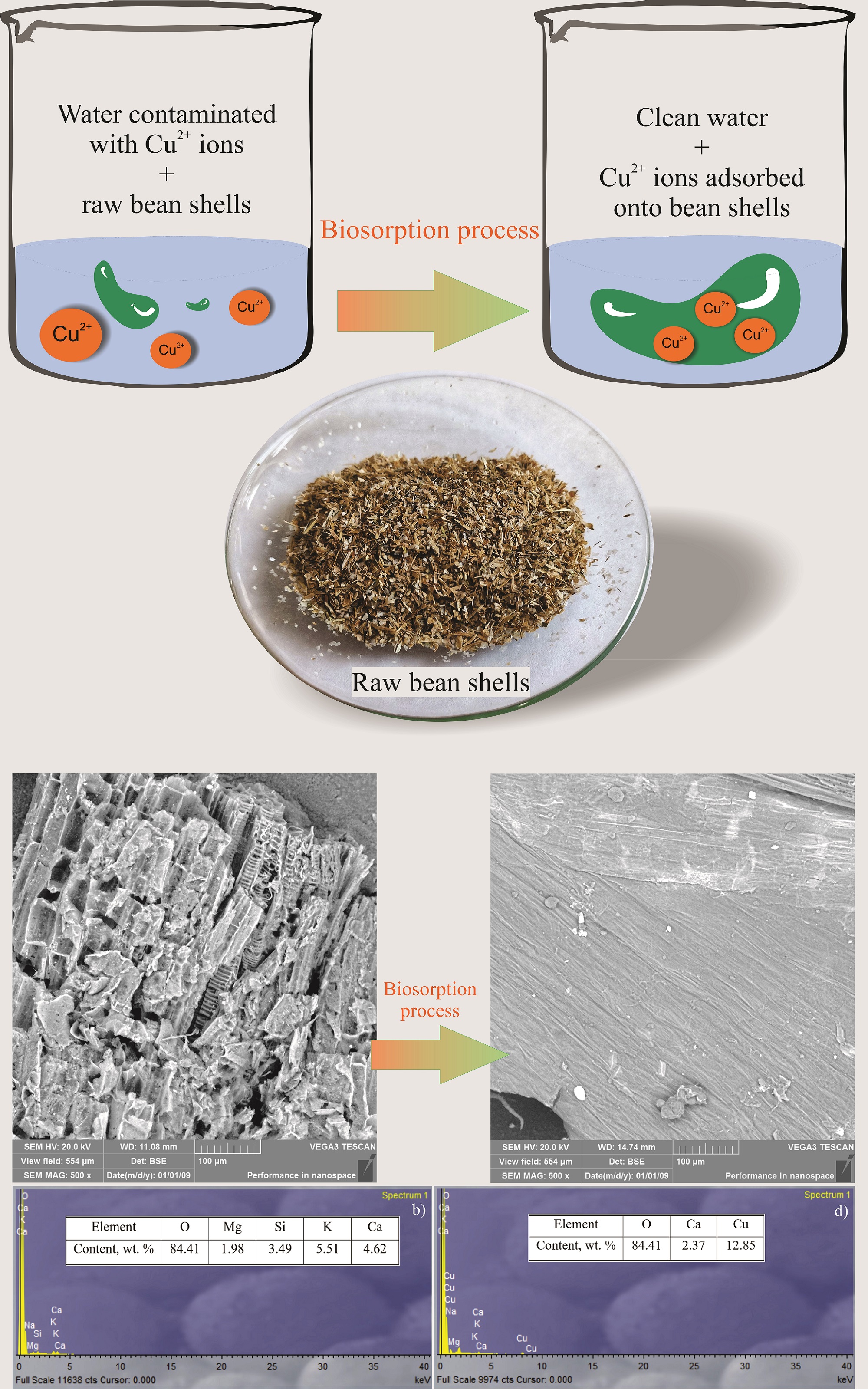
The removal of copper ions from aqueous solutions using bean shells as an adsorbent is presented in this paper. The influence of the solution pH on the biosorption capacity was investigated. The biosorption capacity increased with the increase in the solution pH. The pseudo-second order kinetic model showed the best agreement with the analysed experimental data, indicating that chemisorption could be a possible way of binding the copper ions to the surface of the bean shells. The Langmuir isotherm model best fitted the analysed isotherm data. The SEM-EDS analysis was performed before and after the biosorption process. The change in the morphology of the sample after the biosorption process was evident, whereby K, Mg, Si and Ca were possibly exchanged with copper ions. Response surface methodology (RSM) based on the Box–Behnken design (BBD) was used to optimize the biosorption process, with the selected factors: the solution pH, initial copper ions concentration and contact time. The optimum biosorption conditions were determined to be: pH 3–4, initial copper ions concentration, 100 mg dm-3, and contact time, 10–30 min.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-68/2022-14/200131
References
F. Shafique, Q. Ali, A. Malik, Biol. Clin. Sci. Res. J. 1 (2020) e027 (https://doi.org/10.54112/bcsrj.v2020i1.27)
N. Agasti, CRGSC 4 ( 2021), 100088 (https://doi.org/10.1016/j.crgsc.2021.100088)
D. Lakhterwal, IJERD 4 (2014) 41 (https://www.ripublication.com/ijerd_spl/ijerdv4n1spl_08.pdf)
S. K. Gunatilake, JMESS 1 (2015) 12 (http://www.jmess.org/wp-content/uploads/2015/11/JMESSP13420004.pdf)
C. Tu, Y. Liu, J. Wei, L. Li, K. G. Sheckel, Y. Luo, Environ. Sci. Pollut. Res. Int. 25 (2018) 24965 (https://doi.org/10.1007/s11356-018-2563-4)
M. Marković, M. Gorgievski, D. Božić, V. Stanković, M. Cakić, V. Grekulović, K. Božinović, Rev. Chim. 72 (2021),118 (https://doi.org/10.37358/RC.21.4.8462)
S. Schiewer, B. Volesky, Environ. Sci. Technol. 31 (1997) 2478 (https://doi.org/10.1021/es00012a024)
U. Farooq, J. Kozinski, M. Khan, M. Athar, Bioresour. Technol. 101 (2010) 5043 (https://doi.org/10.1016/j.biortech.2010.02.030)
B. Nagy, C. Manzatu, A. Maicaneanu, C. Indolean, B. T. Lucian, C. Majdik, Arab. J. Chem. 10 (2017) 3569 (https://doi.org/10.1016/j.arabjc.2014.03.004)
S. Lagergren, Sven. Vetenskapsakad. Handingarl 241 (1898) 1
N. T. Coleman, A. C. McClung, D. P. Moore, Science 123 (1956) 330
S. M. Mousa, N. S. Ammar, H. A. Ibrahim, J. Saudi Chem. Soc. 20 (2016) 357 (https://doi.org/10.1016/j.jscs.2014.12.006)
R. S. Juang, M. L. Chen, Ind. Eng. Chem. Res. 36 (1997) 813 (http://dx.doi.org/10.1021/ie960351f)
S. A. Sadeek, N. A. Negm, H. H. Hefni, M. A. Abdel Wahab, Int. J. Biol. Macromol. 81 (2005) 400 (https://doi.org/10.1016/j.ijbiomac.2015.08.031)
R. Han, J. Zhang, W. Zou, J. Shi, H. Liu, J. Hazard. Mater. 125 (2005) 266 (https://doi.org/10.1016/j.jhazmat.2005.05.031)
X. Chen, Information 6 (2015) 14 (https://doi.org/10.3390/info6010014)
G. Murithi, C. O. Onindo, G. K. Muthakia, Bull. Chem. Soc. Ethiop. 26 (2012) 181 (http://dx.doi.org/10.4314/bcse.v26i2.3)
M. Gorgievski, D. Božić, V. Stanković, N. Štrbac, S. Šerbula, Ecol. Eng. 58 (2013) 113 (https://doi.org/10.1016/j.ecoleng.2013.06.025)
D. Božić, V. Stanković, M. Gorgievski, G. Bogdanović, R. Kovačević, J. Hazard. Mater. 171 (2009) 684 (https://doi.org/10.1016/j.jhazmat.2009.06.055)
R. Kumar, H. J. Kumar, M. C. Vishwakarma, H. Sharma, K. S. Joshi, N. S. Bhandari, Environ. Nanotechnol. Monit. Manage. 19 (2023) 100775 (https://doi.org/10.1016/j.enmm.2022.100775)
N. Ilavarasan, Y. S. Sirinivasa Rao, R: Gokulan, A. Aravindan, Glob. Nest J. 25 (2023) 47 (https://doi.org/10.30955/gnj.004496)
M. A. Fawzy, H. M. Al-Yasi, T. M. Galal, R. Z. Hamza, T. G. Abdelkader, E. F. Ali, S. H. A. Hassan, Sci. Rep. 12 (2022) 8583 (https://doi.org/10.1038/s41598-022-12233-1)
C. Sireesha, R. Subha, S. Sumithra, RASAYAN J. Chem. 15 (2022) 2267 (http://doi.org/10.31788/RJC.2022.1548035)
A. Tahir, M. Salman, Desalination Water Treat. 270 (2022) 127 (https://doi.org/10.5004/dwt.2022.28775)
G. F. Coelho, A. C. Goncalves, C. R. Teixeira Tarley, J. Casarin, N. Nacke, M. A. Fancziskowski, Ecol. Eng. 73 (2014) 514 (https://doi.org/10.1016/j.ecoleng.2014.09.103)
A. Choinska-Pulit, J. Sobolczyk-Bednarek, W. Laba, Ecotoxicol. Environ. Saf. 149 (2018) 275 (https://doi.org/10.1016/j.ecoenv.2017.12.008)
H. Turkyilmaz, T. Kartal, S. Yigitarslan Yildiz, J. Environ. Health Sci. Eng. 12 (2014) (https://doi.org/10.1186/2052-336X-12-5).