Structure and properties of ZnO/ZnMn2O4 composite obtained by thermal decomposition of terephthalate precursor Scientific paper
Main Article Content
Abstract
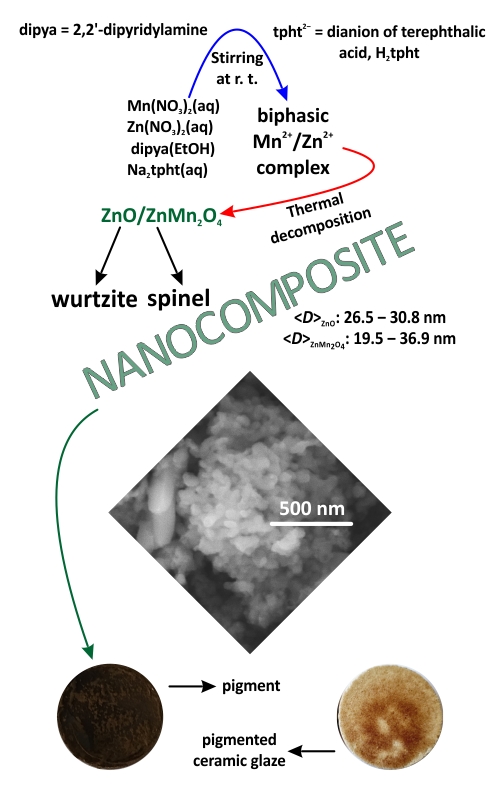
A biphasic [Mn(dipya)(H2O)4](tpht)/{[Zn(dipya)(tpht)]·H2O}n complex material, I (dipya = 2,2’-dipyridylamine, tpht2– = dianion of terephthalatic acid) was synthesized by ligand exchange reaction and characterized by XRPD and FTIR spectroscopy. A ZnO/ZnMn2O4 composite, II, has been prepared via thermal decomposition of I in an air atmosphere at 450 °C. XRPD, FTIR and FESEM analyses of II revealed the simultaneous presence of spherical nanoparticles of wurtzite ZnO and elongated nanoparticles of spinel ZnMn2O4. The specific surface area of II was determined by the BET method, whereas the volume and average size of the mesopores were calculated in accordance with the BJH method. The measurements of the mean size, polydispersity index and zeta potential showed colloidal instability of II. Two band gap values of 2.4 and 3.3 eV were determined using UV–Vis diffuse reflectance spectroscopy, while the measurements of photoluminescence revealed that II is active in the blue region of the visible spectrum. Testing of composite II as a pigmentary material showed that it can be used for the colouring of a ceramic glaze.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-68/2022-14/200135;451-03-68/2022-14/200012;451-03-68/2022-14/200053;451-03-68/2022-14/200287
References
C. Yuan, H. B. Wu, Y. Xie, X. W. Lou, Angew. Chem. Int. Ed. 53 (2014) 1488 (https://dx.doi.org/doi: 10.1002/anie.201303971)
C. N. R. Rao, B. Raveau, Transition Metal Oxides: Structure, Properties, and Synthesis of Ceramic Oxides, 2nd ed., Wiley-VCH, New York, 1998 (https://doi.org/10.1002/(SICI)1099-0739(199906)13:6<476::AID-AOC851>3.0.CO;2-N)
А. Kołodziejczak-Radzimska, T. Jesionowski, Materials 7 (2014) 2833 (https://dx.doi.org/10.3390/ma7042833)
G. D. Park, Y. C. Kang, J. S. Cho, Nanomaterials 12 (2022) 680 (https://doi.org/10.3390/nano12040680)
M. Fortuño-Morte, P. Serna-Gallén, H. Beltrán-Mir, E. Cordoncillo, J. Mat. 7 (2021) 1061 (https://doi.org/10.1016/j.jmat.2021.02.002)
T. E. R. Fiuza, D. Göttert, L. J. Pereira, S. R. M. Antunes, A. V. C. de Andrade, A. C. Antunes, É. C. F. de Souza, Process. Appl. Ceram. 12 (2018) 319 (https://doi.org/10.2298/PAC1804319R)
G. Pfaff, Phys. Sci. Rev. 7 (2022) 7 (https://doi.org/10.1515/psr-2020-0183)
G. Osmond, AICCM Bull. 33 (2012) 20 (http://dx.doi.org/10.1179/bac.2012.33.1.004)
L. J. Almeidaa, E. C. Grzebieluckaa, S. R. M. Antunesa, C. P. F. Borgesa, A. V. C. de Andradeb, É. C. F. de Souza, Mat. Res. 23 (2020) e20190515 (https://doi.org/10.1590/1980-5373-MR-2019-0515)
T. R. Cook, Y. R. Zheng, P. J. Stang, Chem. Rev. 113 (2013) 734 (https://doi.org/10.1021/cr3002824)
D. Sun, R. Cao, Y. Liang, Q. Shi, W. Sua, M. Hong, J. Chem. Soc., Dalton Trans. (2001) 2335 (https://doi.org/10.1039/B102888J)
Z. Cheng, H. Shi, H. Ma, L. Bian, Q. Wu, L. Gu, S. Cai, X. Wang, W. Xiong, Z. An, W. Huang, Angew. Chem. Int. Ed. 57 (2018) 678 (https://doi.org/10.1002/anie.201710017)
C. R. Groom, I. J. Bruno, M. P. Lightfoot, S. C. Ward, Acta Crystallogr., B 72 (2016) 171 (https://doi.org/10.1107/S2052520616003954)
H. Lu, D. S. Wright, S. D. Pike, Chem. Commun. 56 (2020) 854 (https://doi.org/10.1039/C9CC06258K)
M. Y. Masoomi, A. Morsali, Coord. Chem. Rev. 256 (2012) 2921 (https://doi.org/10.1016/j.ccr.2012.05.032)
L. Radovanović, J. D. Zdravković, B. Simović, Ž. Radovanović, K. Mihajlovski, M. D. Dramićanin, J. Rogan, J. Serb. Chem. Soc. 85 (2020) 1475 (https://doi.org/10.2298/JSC200629048R)
L. Radovanović, J. Rogan, D. Poleti, M. V. Rodić, N. Begović, Inorg. Chim. Acta 445 (2016) 46 (https://doi.org/10.1016/j.ica.2016.02.026)
L. Radovanović, J. Rogan, D. Poleti, M. Milutinović, M. V. Rodić, Polyhedron 112 (2016) 18 (https://dx.doi.org/10.1016/j.poly.2016.03.054)
H. M. Rietveld, J. Appl. Cryst. 2 (1969) 65 (https://doi.org/10.1107/S0021889869006558)
J. Rodríguez-Carvajal, Newsletter 26 (2001) 12 (http://journals.iucr.org/iucr-top/comm/cpd/Newsletters/)
M. V. Vasić, L. Pezo, M. R. Vasić, N. Mijatović, M. Mitrić, Bol. Soc. Esp. Ceram. V. (2020) (https://doi.org/10.1016/j.bsecv.2020.11.006)
C. Molinari, S. Conte, C. Zanelli, M. Ardit, G. Cruciani, M. Dondi, Ceram. Int. 46 (2020) 21839 (https://doi.org/10.1016/j.ceramint.2020.05.302)
E. Castellucci, L. Angeloni, N. Neto, G. Sbrana, Chem. Phys. 43 (1979) 365 (https://doi.org/10.1016/0301-0104(79)85204-0)
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Organic Coordination Compounds, Part B, 5th ed., Wiley-Interscience, New York, 1997
N. Senthilkumar, V. Venkatachalam, M. Kandiban, P. Vigneshwaran, R. Jayavel, Vetha Potheher, Physica E 106 (2019) 121 (https://doi.org/10.1016/j.physe.2018.10.027)
W. Konicki, D. Sibera, U. Narkiewicz, Separ. Sci. Technol. 53 (2018) 1295 (https://doi.org/10.1080/01496395.2018.1444054)
Holmberg, D. O. Shah, M. J. Schwuger, Handbook of Applied Surface and Colloid Chemistry, Vol. 2, John Wiley & Sons, Ltd., Chichester, 2002
R Greenwood, K Kendall, J. Eur. Ceram. Soc. 19 (1999) 479 (http://dx.doi.org/10.1016/S0955-2219(98)00208-8)
M. Staiger, P. Bowen, J. Ketterer, J. Bohonek, J. Disper. Sci. Technol. 23 (2002) 619 (https://doi.org/10.1081/DIS-120015367)
Nie, G. Chang, R. Li, Coatings 20 (2020) 741 (https://doi.org/10.3390/coatings10080741)
H. Morii, K. Hayashi, K. Iwasaki, (Hiroshima-shi, Hiroshima-ken (JP)), EP 1 686 158 B1 (2006)
E. A. Medina, J. Li, M. A. Subramanian, Prog. Solid State Chem. 45–46 (2017) 9 (https://doi.org/10.1016/j.progsolidstchem.2017.02.002)
SRPS EN ISO 10545-15: Ceramic tiles — Part 15: Determination of lead and cadmium given off by glazed tiles, 2012
J. W. Gallaway, M. Menard, B. Hertzberg, Z. Zhong, M. Croft, L. A. Sviridov, D. E. Turney, S. Banerjee, J. Electrochem. Soc. 162 (2015) A162 (https://doi.org/10.1149/2.0811501jes).