The influence of conversion creatine and guanidinoacetic acid from zwitterionic to cationic form on their solubility in water - a thermodynamic study
Main Article Content
Abstract
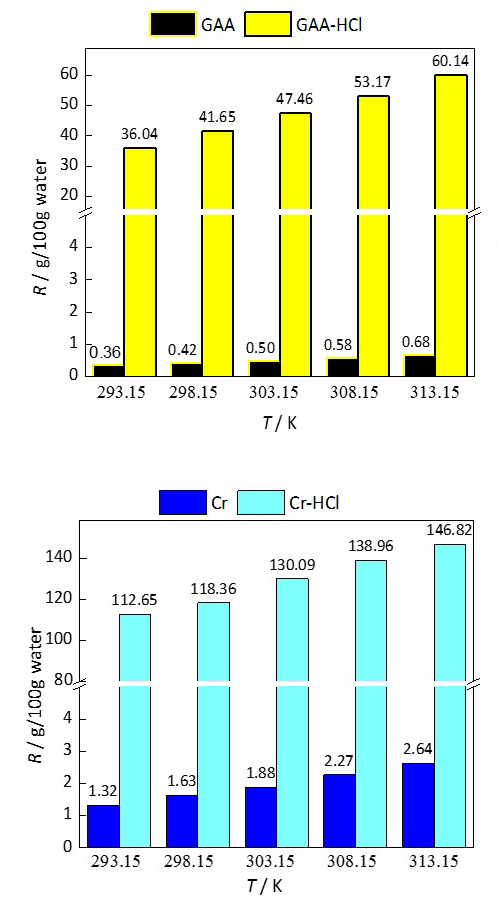
In this work, the solubility of creatine, creatinine, guanidinoacetic acid, and their hydrochlorides in water at atmospheric pressure and in the temperature range T = (293.15 - 313.15) K was determined by the gravimetric method. The thermodynamic parameters of dissolution in water for the mentioned compounds were calculated. The solubility increases significantly by converting the zwitterionic structures of creatine and guanidinoacetic acid into a cationic form, i.e. hydrochloride salt. The effect of increasing solubility is more pronounced for guanidinoacetic acid and decreases with temperature for both compounds. A simple process of transforming electrically neutral zwitterionic structures into cations represents a good way to increase the solubility in water and bioavailability of biologically active compounds.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Provincial Secretariat for Higher Education and Scientific Research, Autonomous Province of Vojvodina
Grant numbers 142-451-2545/2021-01
References
R. Cooper, F. Naclerio, J. Allgrove, A. Jimenez, J. Int. Soc. Sports Nutr. 9 (2012) 33 (https://doi.org/10.1186/1550-2783-9-33)
R. Jäger, M. Purpura, A. Shao, T. Inoue, R. B. Kreider, Amino Acids 40 (2011) 1369 (https://doi.org/10.1007/s00726-011-0874-6)
S. Ostojić, B. Niess, M. Stojanović, M. Obrenović, Int. J. Medical Sci. 10 (2013) 141 (https://doi.org/10.7150/ijms.5125)
S. Ostojić, M. Stojanović, P. Drid, J. R. Hoffman, D. Sekulić, N. Zenić. Nutrients 8 (2016) 72 (https://doi.org/10.3390/nu8020072)
S. C. Forbes, D. M. Cordingley, S. M. Cornish, B. Gualano, H. Roschel, S. M. Ostojić, E. S. Rawson, B.D. Roy, K. Prokopidis, P. Giannos, D.G. Candow, Nutrients 14 (2022) 921 (https://doi.org/10.3390/nu14050921)
M. Vraneš, S. Ostojić, A. Tot, S. Papović, S. Gadžurić, Food Chem. 237 (2017) 53 (https://doi.org/10.1016/j.foodchem.2017.05.088)
C. M. Romero, C. D. Oviedo, J. Solution Chem. 6 (2013) 1355 (https://doi.org/10.1007/s10953-013-0031-9)
M. Jaffe, Biol. Chem. 10 (1886) 391 (https://doi.org/10.1515/bchm1.1886.10.5.391)
M. Vraneš, S. Papović, Creatine in sport: Creatine supplementation, in Human health and nutrition: New forms of creatine in human nutrition, S. Ostojić, Nova Science Publishers, Nova Biomedical, New York, USA, 2015, p. 105 (https://open.uns.ac.rs/handle/123456789/5425)
J. Sha, T. Ma, R. Zhao, P. Zhang, R. Sun, G. Jiang. Y. Wan, H. He, X. Yao, Y. Li, T. Li, B. Ren, J. Chem. Thermodyn. 144 (2020) 106073 (https://doi.org/10.1016/j.jct.2020.106073)
F. Shakeel, M. Imran, N. Haq, S. Alshehri, M. K. Anwer, Molecules 24 (2019) 3404 (https://doi.org/10.3390/molecules24183404)
M. Vraneš, S. Ostojić, Č. Podlipnik, A. Tot, J. Chem. Res. 45 (2021) 467 (https://doi.org/10.1177/1747519820978583).