DNA/BSA interactions and cytotoxic studies of tetradentate N,N,O,O-Schiff base copper(II) complexes Scientific paper
Main Article Content
Abstract
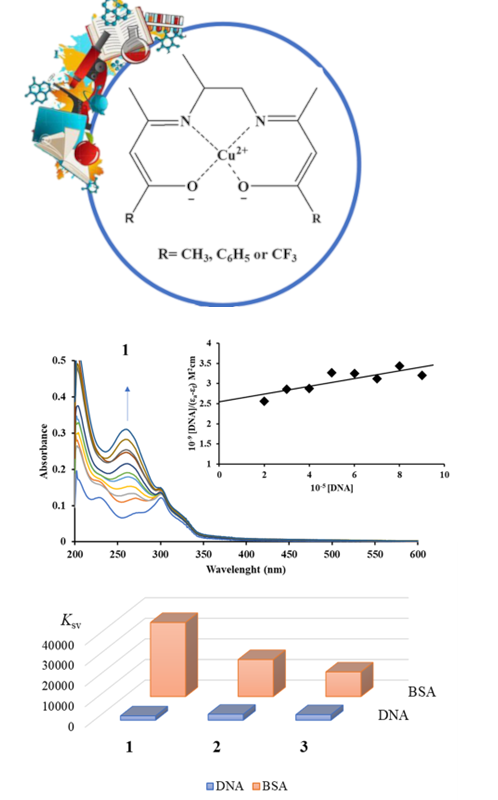
Three Schiff base Cu(II) complexes, (N,N’-bis(acetylacetone)propylenediimine)copper(II) complex, [Cu(acac2pn)] (1), (N,N'-bis-(benzoylacetone)propylenediimine)copper(II) complex, [Cu(phacac2pn)] (2) and (N,N’-bis-(trifluoroacetylacetone)propylenediimine)copper(II) complex, [Cu(tfacac2pn)] (3), were used to investigate the interactions with calf thymus DNA (ct-DNA) and bovine serum albumin (BSA) using the electronic absorption and spectroscopic fluorescence methods. UV–Vis absorption studies showed that studied complexes interact with DNA molecule and exhibit moderate binding affinity. Fluorescence studies of complexes 1–3 also showed a possibility for DNA intercalation as well as a relatively high binding ability toward BSA. Among the tested complexes, the highest affinity for DNA and BSA molecules was shown by complex 1. Cytotoxic analyses, performed on human colorectal carcinoma HCT-116 and healthy lung fibroblast MRC-5 cell lines, showed that complex 2 exhibited activity on both cell lines, while complexes 1 and 3 did not show any activity.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/ 200122, 451-03-47/2023-01/200126, 451-03-47/2023-01/200126, 451-03-47/2023-01/200125
References
J. C. Dabrowiak, Metals in Medicine, 2nd ed., Wiley, New York, 2017 (ISBN:9781119191377, (https://doi.org/10.1002/9781119191377))
S. Medicia, M. Peanaa, V. M. Nurchib, J. I. Lachowiczb, G. Crisponib, M. A. Zoroddua, Coord. Chem. Rev. 284 (2015) 329 (https://doi.org/10.1016/j.ccr.2014.08.002)
F. Tistao, C. Marzano, M. Porchia, M. Pellei, C. Santini, Med. Res. Rev. 30 (2010) 708 (https://doi.org/10.1002/med.20174)
C. Marzano, M. Pellei, F. Tristao, C. Santini, Anticancer Agents Med. Chem. 9 (2009) 185 (https://doi.org/10.2174/187152009787313837)
M. Karmakar, S. Chattopadhyay, J. Mol. Struct. 1186 (2019) 155 (https://doi.org/10.1016/J.MOLSTRUC.2019.02.091)
C. Boulechfar, H. Ferkous, A. Delimi, A. Djedouani, A. Kahlouche, A. Boublia, A.S. Darwish, T. Lemaoui, R. Verma, Y. Benguerba, Inorg. Chem. Commun. 150 (2023) 110451 (https://doi.org/10.1016/j.inoche.2023.110451)
S. Yamada, Coord. Chem. Rev. 1 (1966) 415 (https://doi.org/10.1016/S0010-8545(00)80184-8)
L. H. A. Rahman, R. M. E. Khatib, L. A. E. Nassr, A. M. A. Dief, F. E. Lashin, Spectrochim. Acta, A 111 (2013) 266 (https://doi.org/10.1016/j.saa.2013.03.061)
L. H. A. Rahman, R. M. E. Khatib, L. A. E. Nassr, A. M. A. Dief, A. A. Seleem, Spectrochim. Acta, A 117 (2014) 366 (https://doi.org/10.1016/j.saa.2013.07.056)
X. G. Ran, L. Y. Wang, Y. C. Lin, J. Hao, D. R. Cao, Appl. Organometal. Chem. 24 (2010) 741 (https://doi.org/10.1002/aoc.1678)
F. Zhao, W. Wang, W. Lu, L. Xu, S. Yang, X. Cai, M. Zhou, M. Lei, M. Ma, H. Xu, F. Cao, Eur. J. Med. Chem. 146 (2018) 451 (https://doi.org/10.1016/j.ejmech.2018.01.041)
R. Fekri, M. Salehi, A. Asadi, M. Kubicki, Inorg. Chim. Acta 484 (2018) 245 (https://doi.org/10.1016/J.ICA.2018.09.022)
L. N. Ji, X. H. Zou, J. G. Liu, Coord. Chem. Rev. 216 (2001) 513 (https://doi.org/10.1016/S0010-8545(01)00338-1)
A. Nori, J. Kopecek, Adv. Drug Delivery Rev. 57 (2005) 609 (https://doi.org/10.1016/j.addr.2004.10.006)
R. K. Gupta, R. Pandey, G. Sharma, R. Prasad, B. Koch, S. Srikrishna, P. Z. Li, Q. Xu, D. S. Pandey, Inorg. Chem. 52 (2013) 3687 (https://doi.org/10.1021/ic302196v)
M. Ganeshpandian, R. Loganathan, E. Suresh, A. Riyasdeen, M. A. Akbarshad, M. Palaniandavar, Dalton Trans. 43 (2014) 1203 (https://doi.org/10.1039/C3DT51641E)
R. Baosic, D. Milojkovic-Opsenica, Z. Tesic, J. Planar Chromatogr. – Mod. TLC 16 (2003) 412 ( https://doi.org/10.1556/JPC.15.2002.4.4)
P. J. Mc Carthy, R. J. Hovey, K. Ueno, A. E. Martel, J. Am. Chem. Soc. 77 (1955) 5820 (https://doi.org/10.1021/ja01627a011)
N. Stevanovic, D. Apostolovic, M. Milcic, A. Lolic, M. van Hage, T. Cirkovic Velickovic, R. Baosic, New J. Chem. 45 (2021) 6231 (https://doi.org/10.1039/d1nj00040c)
F. Dimiza, S. Fountoulaki, A. N. Papadopoulos, C. A. Kontogiorgis, V. Tangoulis, C. P. Raptopoulou, V. Psycharis, A. Terzis, D. P. Kessissoglou, G. Psomas, Dalton Trans. 40 (2011) 8555 (https://doi.org/10.1039/c1dt10714c)
F. Dimiza, F. Perdih, V. Tangoulis, I. Turel, D. P. Kessissoglou, G. Psomas, J. Inorg. Biochem. 105 (2011) 476 (https://doi.org/10.1016/j.jinorgbio.2010.08.013)
P. Čanović, J. Bogojeski, J.V. Košarić, S.D. Marković, M.N. Živanović. Turk. J. Biol. 41 (2017) 141 ( https://doi.org/10.3906/BIY-1605-77)
J. V. Košarić, D. M. Cvetković, M. N. Živanović, M. G. Ćurčić, D. S. Šeklić, Z. M. Bugarčić, S. D. Marković. J. Buon. 19 (2014) 283 (https://jbuon.com/archive/19-1-283.pdf)
A. Petrović, M. M. Milutinović, E. T. Petri, M. Živanović, N. Milivojević, R. Puchta, A. Scheurer, J. Korzekwa, O. R. Klisurić, J. Bogojeski, Inorg. Chem. 58 (2019) 307 (https://doi.org/10.1021/acs.inorgchem.8b02390)
O. Novakova, H. Chen, O. Vrana, A. Rodger, P. J. Sadler, V. Brabec, Biochemistry 42 (2003) 11544 (https://doi.org/10.1021/bi034933u)
E. S. Koumousi, M. Zampakou, C. P. Raptopoulou, V. Psycharis, C. M. Beavers, S. J. Teat, G. Psomas and T. C. Stamatatos, Inorg. Chem. 5 (2012) 7699 (https://doi.org/10.1021/ic300739x)
M. M. Milutinović, J. V. Bogojeski, O. Klisurić, A. Scheurer, S. K. C. Elmroth, Ž. D. Bugarčić, Dalton Trans. 45 (2016) 15481 (https://doi.org/10.1039/c6dt02772e)
J. M. Kelly, A. B. Tossi, D. J. McConnell, C. Oh Uigin, Nucleic Acids Res. 13 (1985) 6017 (https://doi.org/10.1093/nar/13.17.6017)
B. C. Boger, B. E. Fink, S. R. Brunette, W. C. Tse, M. P. Hedrick, J. Am. Chem. Soc. 123 (2001) 5878 (https://doi.org/10.1021/ja010041a)
D. Senthil Raja, N. S. P. Bhuvanesh, K. Natarajan, Inorg. Chem. 50 (2011) 12852 (https://doi.org/10.1021/ic2020308 )
J. Steinhardt, J. Krijn and J. G. Leidy, Biochemistry 10 (1971) 4005 (https://doi.org/10.1021/bi00798a001)
Y. Song, Y. Liu, W. Liu, F. A. Villamena and J. L. Zweier, RSC Adv. 4 (2014) 47649 (https://doi.org/10.1039/C4RA04616A)
F. Wang, W. Huang, Z. Dai, J. Mol. Struct. 875 (2008) 509 (https://doi.org/10.1016/j.molstruc.2007.05.034)
A. Mijatović, N. Gligorijevic, D. Cocic, S. Spasic, A. Lolić, S. Aranđelović, M. Nikolić, R. Baošić, J. Inorg. Biochem. 244 (2023) 112224 (https://doi.org/10.1016/j.jinorgbio.2023.112224).