Assignment of NMR spectral data of diastereomeric tetrahydrofuranyl acetals directly from their mixture by spectral simulation Scientific paper
Main Article Content
Abstract
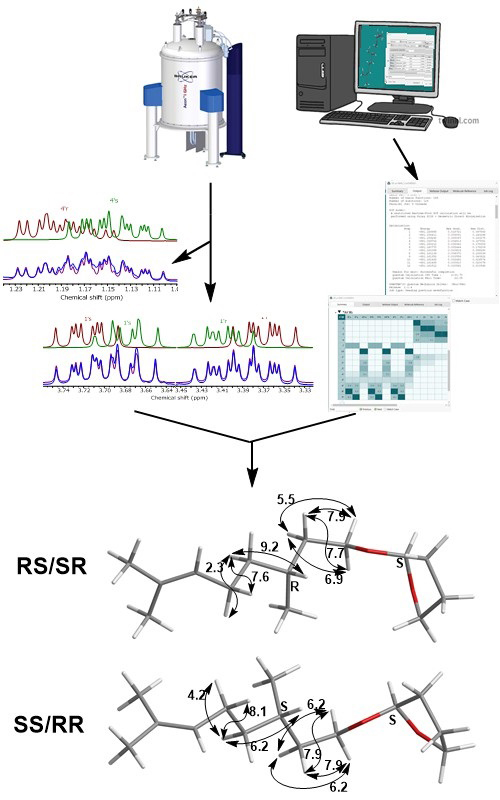
In this study, an NMR spectral analysis of the mixture of diastereomeric acetals, synthesized from 2,3-dihydrofurane and a racemic mixture of citronellol, was performed. 1H-NMR full spin analysis was achieved by manually adjusting δH and J values (previously calculated using the Spartan software) to fit the experimentally available values, followed by further optimization using MestreNova software. The simulated 1H- and 13C-NMR spectra of individual diastereomers, as well as their superimposed and summed spectra, were compared with the obtained experimental spectra. Spin simulation of proton signals was particularly useful for the assignment of the diastereotopic protons of tetrahydrofuranyl moiety and diastereomer discrimination. The NMR spectral data of individual diastereomers – chemical shifts, coupling constants, HMBC and NOESY interactions were systematized in appropriate tables and schemes. To the best of our knowledge, this is for the first time that the complete assignment of tetrahydrofuranyl moiety was performed, and the data obtained herein may be of great importance for the utilization of this protecting group in the future.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200124
References
P. G. M. Wuts, T. W. Greene, Greene’s Protective Groups in Organic Synthesis, John Wiley & Sons, Inc., Hoboken, NJ, 2006 (https://dx.doi.org/10.1002/0470053488)
B. Kumar, M. A. Aga, A. Rouf, B. A. Shah, S. C. Taneja, RSC Adv. 4 (2014) 21121 (https://dx.doi.org/10.1039/c4ra02093f )
N. S. Radulović, M. S. Nešić, RSC Adv. 6 (2016) 93068 (https://dx.doi.org/10.1039/C6RA19980A)
M. Barbero, S. Bazzi, S. Cadamuro, S. Dughera, C. Piccinini, Synthesis 2 (2010) 315 (https://dx.doi.org/10.1055/s-0029-1217093)
H. Fujioka, T. Okitsu, T. Ohnaka, R. Li, O. Kubo, K. Okamoto, Y. Sawama, Y. Kita, J. Org. Chem. 72 (2007) 7898 (https://dx.doi.org/10.1021/jo071187g)
C. V. T. Vo, T. A. Mitchell, J. W. Bode, J. Am. Chem. Soc. 133 (2011) 14082 (https://dx.doi.org/10.1021/ja205174c)
L. S. Li, S. Das, S. C. Sinha, Org. Lett. 6 (2004) 127 (https://dx.doi.org/10.1021/ol030108u)
S. Yoshioka, M. Oshita, M. Tobisu, N. Chatani, Org. Lett. 7 (2005) 3697 (https://dx.doi.org/10.1021/ol0513138)
L. Lemiègre, R. L. Stevens, J. C. Combret, J. Maddaluno, Org. Biomol. Chem. 3 (2005) 1308 (https://dx.doi.org/10.1039/B419381D)
A. Fürstner, T. Gastner, Org. Lett. 2 (2000) 2467 (https://dx.doi.org/10.1021/ol0061236)
A. Robinson, V. K. Aggarwal, Angew. Chem. Int. Ed. 49 (2010) 6673 (https://dx.doi.org/10.1002/anie.201003236)
N. Hama, T. Matsuda, T. Sato, N. Chida, Org. Lett. 11 (2009) 2687 (https://dx.doi.org/10.1021/ol900799e)
E. L. Eliel, B. E. Nowak, R. A. Daignault, V. G. Badding, J. Org. Chem. 30 (1962) 2441 (https://dx.doi.org/10.1021/jo01018a082)
L. J. Lambert, M. J. Miller, P. W. Huber, Org. Biomol. Chem. 13 (2015) 2341 (https://dx.doi.org/10.1039/C4OB02212B)
E. J. Corey, N. M. Weinshenker, T. K. Schaaf, W. Huber, J. Am. Chem. Soc. 91 (1969) 5675 (https://dx.doi.org/10.1021/ja01048a062)
H. Kusama, R. Hara, S. Kawahara, T. Nishimori, H. Kashima, N. Nakamura, K. Morihira, I. Kuwajima, J. Am. Chem. Soc. 122 (2000) 3811 (https://dx.doi.org/10.1021/ja9939439)
T. Mukaiyama, I. Shiina, H. Iwadare, M. Saitoh, T. Nishimura, N. Ohkawa, H. Sakoh, K. Nishimura, Y.-I. Tani, M. Hasegawa, K. Yamada, K. Saitoh, Chem. Eur. J. 5 (1991) 121 (https://dx.doi.org/10.1002/(SICI)1521-3765(19990104)5:1<121::AID-CHEM121>3.0.CO;2-O)
J. D. Winkler, M. B. Rouse, M. F. Greaney, S. J. Harrison, Y. T. Jeon, J. Am. Chem. Soc. 124 (2002) 9726 (https://dx.doi.org/10.1021/ja026600a)
G. Stork, A. Yamashita, J. Adams, G. R. Schulte, R. Chesworth, Y. Miyazaki, J. J. Farmer, J. Am. Chem. Soc. 131 (2009) 11402 (https://dx.doi.org/10.1021/ja9038505)
B. Liu, S. Thayumanavan, J. Am. Chem. Soc. 139 (2017) 2306 (https://dx.doi.org/10.1021/jacs.6b11181)
S. J. Danishefsky, J. J. Masters, W. B. Young, J. T. Link, L. B. Snyder, T. V Magee, D. K. Jung, R. C. A. Isaacs, W. G. Bornmann, C. A. Alaimo, C. A. Coburn, M. J. di Grandi, J. Am. Chem. Soc. 118 (1996) 2843 (https://dx.doi.org/10.1021/ja952692a)
I. Ramos-Tomillero, H. Rodriguez, F. Albericio, Org. Lett. 17 (2015) 1680 (https://dx.doi.org/10.1021/acs.orglett.5b00444)
23. A. Sharma, I. Ramos-Tomillero, A. El-Faham, E. Nicolas, H. Rodriguez, B. G. de la Torre, F. Albericio, ChemistryOpen 6 (2017) 168 (https://dx.doi.org/10.1002/open.201600156)
P. L. Santos, J. P. S. C. F. Matos, L. Picot, J. R. G. S. Almeida, J. S. S. Quintans, L. J. Quintans-Júnior, Food Chem. Toxicol. 123 (2019) 459 (https://dx.doi.org/10.1016/j.fct.2018.11.030)
W. S. Hsu, J. H. Yen, Y. S. Wang, J. Environ. Sci. Health., B 48 (2013) 1014 (https://dx.doi.org/10.1080/03601234.2013.816613)
N. Monnerie, J. Ortner, J. Sol. Energy Eng. 123 (2001) 171 (https://dx.doi.org/10.1115/1.1354996)
Jozef Kula, Aleksandra Wojciechowska, PL224652 (2017)
N. S. Radulović, S. I. Filipović, M. S. Nešić, N. M. Stojanović, K. V. Mitić, M. Z. Mladenović, V. N. Randelović, J. Nat. Prod. 83 (2020) 3554 (https://dx.doi.org/10.1021/acs.jnatprod.0c00585)
N. S. Radulović, M. Z. Mladenović, N. M. Stojanović, P. J. Randjelović, P. D. Blagojević, J. Nat. Prod. 82 (2019) 1874 (https://dx.doi.org/10.1021/acs.jnatprod.9b00120)
N. Radulović, M. Stevanović, M. Nešić, N. Stojanović, P. Ranelović, V. Ranelović, J. Nat. Prod. 83 (2020) 2902 (https://dx.doi.org/10.1021/acs.jnatprod.0c00437)
M. Nesic, N. Radulovic, Facta Univ., Ser.: Phys., Chem. Technol. 19 (2021) 69 (https://dx.doi.org/10.2298/FUPCT2102069N)
H. Gunther, NMR Spectroscopy; Basic Principles, Concepts and Application in Chemistry Third Edition, Wiley-VCH, Weinheim, 2013 (ISBN: 978-3-527-33000-3)
C. A. G. Haasnoot, F. A. A. M. de Leeuw, C. Altona, Tetrahedron 36 (1980) 2783 (https://dx.doi.org/10.1016/0040-4020(80)80155-4)
L. A. Donders, F. A. A. M. De Leeuwt, C. Altonaz, Magn. Reson. Chem. 27 (1989) 556 (https://dx.doi.org/10.1002/mrc.1260270608).