Diorganotin(IV) complexes with hydroxamic acids derivatives of some histone deacetylases inhibitors Scientific paper
Main Article Content
Abstract
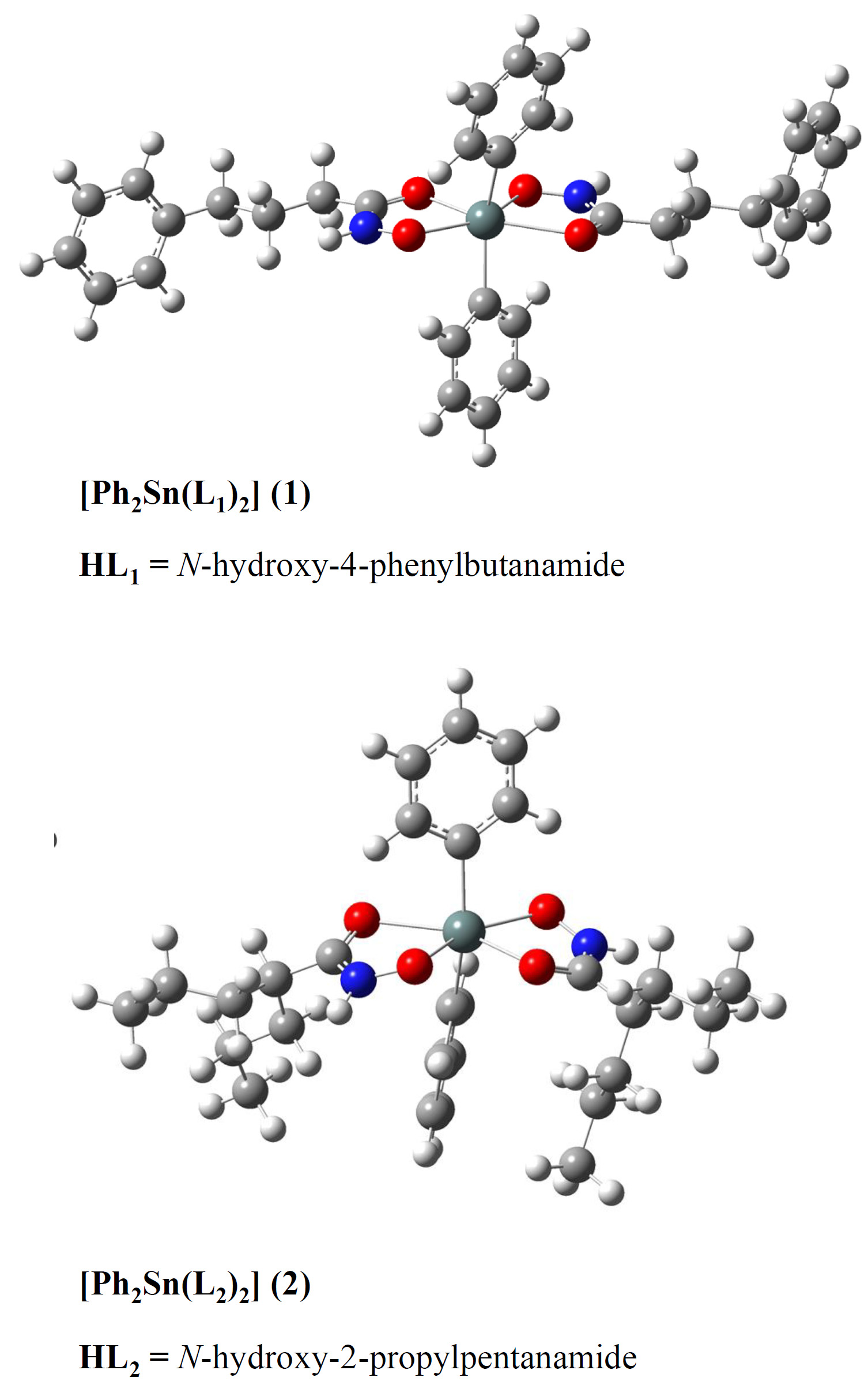
Organotin(IV) compounds show great potential as antitumor metallodrugs with lower toxicity and higher antiproliferative activity. Histone deacetylases (HDAC) inhibitors are characterised by high bioavailability and low toxicity. In this research, the two novel octahedral organotin(IV) complexes of physiologically active hydroxamate-based ligands, N-hydroxy-4-phenylbutanamide (HL1) and N-hydroxy-2-propylpentanamide (HL2), have been prepared and characterized using FTIR, 1H-, 13C- and 119Sn-NMR spectroscopy. Particular emphasis was put on the binding characteristics of ligands. The structures were additionally analysed by the density functional theory at B3LYP-D3BJ/6-311++G(d,p)(H,C,N,O)/LanL2DZ(Sn) level. The theoretical IR and NMR spectra were compared to the spectroscopic data, and it was concluded that the predicted structures described well the experimental ones. The stability of different isomers of HL1 and HL2 was assessed by the natural bond orbital analysis, and the importance of intramolecular hydrogen bond was outlined. The interactions between donor atoms and Sn were investigated and correlated with the changes in chemical shift and the wavenumbers of characteristic vibrations.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200124 and 451-03-47/2023-01/200146
References
J. Chen, D. Faller, R. Spanjaard, Curr. Cancer Drug Targets 3 (2003) 219 (https://doi.org/10.2174/1568009033481994)
G. Li, Y. Tian, W.-G. Zhu, Front. Cell Dev. Biol. 8 (2020) (https://doi.org/10.3389/fcell.2020.576946)
J. L. Tischler, B. Abuaita, S. C. Cuthpert, C. Fage, K. Murphy, A. Saxe, E. B. Furr, J. Hedrick, J. Meyers, D. Snare, A. R. Zand, J. Enzyme Inhib. Med. Chem. 23 (2008) 549 (https://doi.org/10.1080/14756360701715703)
D. M. Fass, R. Shah, B. Ghosh, K. Hennig, S. Norton, W.-N. Zhao, S. A. Reis, P. S. Klein, R. Mazitschek, R. L. Maglathlin, T. A. Lewis, S. J. Haggarty, ACS Med. Chem. Lett. 2 (2011) 39 (https://doi.org/10.1021/ml1001954)
J. Devi, M. Yadav, D. K. Jindal, D. Kumar, Y. Poornachandra, Appl. Organomet. Chem. 33 (2019) 1 (https://doi.org/10.1002/aoc.5154)
S. N. Syed Annuar, N. F. Kamaludin, N. Awang, K. M. Chan, Front. Chem. 9 (2021) (https://doi.org/10.3389/fchem.2021.657599)
S. Hadjikakou, N. Hadjiliadis, Coord. Chem. Rev. 253 (2009) 235 (https://doi.org/10.1016/j.ccr.2007.12.026)
X. Shang, E. C. B. A. Alegria, M. F. C. Guedes da Silva, M. L. Kuznetsov, Q. Li, A. J. L. Pombeiro, J. Inorg. Biochem. 117 (2012) 147 (https://doi.org/10.1016/j.jinorgbio.2012.08.019)
S. Kumari, N. Sharma, J. Coord. Chem. 72 (2019) 584 (https://doi.org/10.1080/00958972.2019.1573993)
V. K. Choudhary, A. K. Bhatt, N. Sharma, J. Coord. Chem. 72 (2019) 372 (https://doi.org/10.1080/00958972.2019.1573993)
N. Naoom, E. Yousif, D. S. Ahmed, B. M. Kariuki, G. A. El-Hiti, Polymers 14 (2022) 4590 (https://doi.org/10.3390/polym14214590)
R. R. Arraq, A. G. Hadi, D. A. Ahmed, G. A. El-Hiti, B. M. Kariuki, A. A. Husain, M. Bufaroosha, E. Yousif, Polymers 15 (2023) 550 (https://doi.org/10.3390/polym15030550)
R. Haddad, S. Khadum, M. Ali, A. Majeed, A. Husain, M. Bufaroosha, D. Ahmed, E. Yousif, Bull. Chem. Soc. Ethiop. 37 (2023) 771 (https://dx.doi.org/10.4314/bcse.v37i3.18)
M. S. Genčić, N. M. Stojanović, M. Z. Mladenović, N. S. Radulović, Neurochem. Int. 161 (2022) 105433 (https://doi.org/10.1016/j.neuint.2022.105433)
U. Gravemann, J. Volland, H. Nau, Neurotoxicol. Teratol. 30 (2008) 390 (https://doi.org/10.1016/j.ntt.2008.03.060)
A. D. Becke, Phys. Rev., A 38 (1988) 3098 (https://doi.org/10.1103/PhysRevA.38.3098)
A.D. Becke, E.R. Johnson, J. Chem. Phys. 123 (2005) 154101 (https://doi.org/10.1063/1.2065267)
T. H. Dunning, J. Chem. Phys. 90 (1989) 1007 (https://doi.org/10.1103/10.1063/1.456153)
P. J. Hay, W. R. Wadt, J. Chem. Phys. 82 (1985) 299 (https://doi.org/10.1063/1.448975)
P. J. Hay, W. R. Wadt, J. Chem. Phys. 82 (1985) 270 (https://doi.org/10.1063/1.448799)
Gauss View, Version 5, Semichem Inc., Shawnee, KS, 2009
A. V. Marenich, C. J. Cramer, D. G. Truhlar, J. Phys. Chem., B 113 (2009) 6378 (https://doi.org/10.1021/jp810292n)
J. A. Bohmann, F. Weinhold, T. C. Farrar, J. Chem. Phys. 107 (1997) 1173 (https://doi.org/10.1063/1.474464)
J. P. Foster, F. Weinhold, J. Am. Chem. Soc. 102 (1980) 7211 (https://doi.org/10.1021/ja00544a007)
D. A. Brown, W. K. Glass, R. Mageswaran, S. A. Mohammed, Magn. Reson. Chem. 29 (1991) 40 (https://doi.org/10.1002/mrc.1260290109)
B. García, S. Ibeas, J. M. Leal, F. Secco, M. Venturini, M. L. Senent, A. Niño, C. Muñoz, Inorg. Chem. 44 (2005) 2908 (https://doi.org/10.1021/ic049438g)
D. A. Brown, R. A. Coogan, N. J. Fitzpatrick, W. K. Glass, D. E. Abukshima, L. Shiels, M. Ahlgrén, K. Smolander, T. T. Pakkanen, T. A. Pakkanen, M. Peräkylä, J. Chem. Soc., Perkin Trans. 2 (1996) 2673 (https://doi.org/10.1039/P29960002673)
J. Adeyemi, D. Onwudiwe, Molecules 23 (2018) 2571 (https://doi.org/10.3390/molecules23102571)
D. S. Dimić, Z. S. Marković, L. Saso, E. H. Avdović, J. R. Đorović, I. P. Petrović, M. J. Stanisavljević, D. D. Stevanović, I. Potočňák, E. Samoľová, S. R. Trifunović, J. M. Dimitrić Marković, Oxid. Med. Cell. Longev. 2019 (2019) 2069250 (https://doi.org/10.1155/2019/2069250)
T. Eichhorn, F. Kolbe, S. Mišić, D. Dimić, I. Morgan, M. Saoud, D. Milenković, Z. Marković, T. Rüffer, J. Dimitrić Marković, G. N. Kaluđerović, Int. J. Mol. Sci. 24 (2023) (https://doi.org/10.3390/ijms24010689).