Synthesis of poly(itaconic acid) and its application for synthesis of pyrrolinones as a reusable homogeneous catalyst Scientific paper
Main Article Content
Abstract
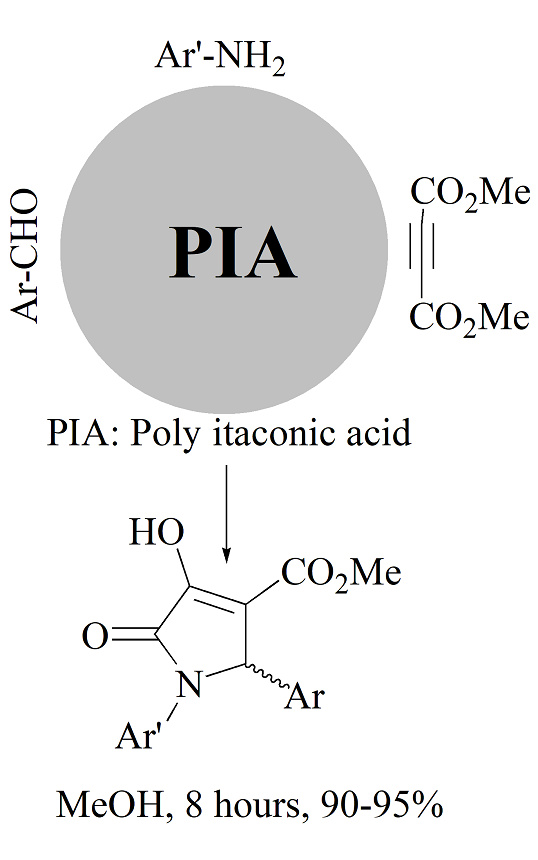
In this context, poly(itaconic acid) (PIA) was synthesized through the polymerization of itaconic acid in the presence of K2S2O8 and NaH2PO4, achieving satisfactory yields of 80 % within 48 h at 55 °C. The PIA served as a homogeneous catalyst in MeOH for the synthesis of pyrrolin-2-ones from aromatic aldehydes, anilines and dimethyl acetylenedicarboxylate (DMAD). The reactions proceeded efficiently, yielding excellent results with conversion rates of 90–95 % within 8 h at room temperature. The synthesized products were characterized by combustion analysis (CHN), 1H-NMR, 13C-NMR and FT-IR spectra. This method is eco-friendly and aligns with the principles of green chemistry.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
H. Wang, N. Li, J. Zang, X. Wan, Chirality 27 (2015) 523 (https://doi.org/10.1002/chir.22448)
N. Haque, S. Biswas, P. Basu, I. Haque Biswas, R. khatun, A. Khan, S. M. Islam, New J. Chem. 44 (2020) 15446 (https://doi.org/10.1039/D0NJ02798G)
P. Aguirre, K. Brown, D. Venegas-Yazigi, V. Paredes-Garcıa, E. Spodine, Macromol. Symp. 304 (2011) 65 (https://doi.org/10.1002/masy.201150609)
M. Najafi, A. Abbasi, M. Masteri-Farahani, H. Shahbaazi, M. Ahmadniai Motlagh, J. Janczak, RSC Adv. 6 (2016) 29944 (https://doi.org/10.1039/C6RA02248K)
P. Puthiaraja, Y. M. Chung, W. S. Ahna, Mol. Catal. 441 (2017) 1 (https://doi.org/10.1016/j.mcat.2017.08.002)
J. J. Kim, C-R, Lim, B. M. Reddy, S. E. Park, Mol. Catal. 451 (2018) 43 (https://doi.org/10.1016/j.mcat.2017.10.022)
G. Fan, H. Zhang, S. Cheng, Z. Ren, Z. Hu, Z. Wang, Aust. J. Chem. 61 (2008) 610 (https://doi.org/10.1071/CH08066)
Y. Tian, R. Zhang, W. Zhao, S. Wen, Y. Xiang, X. Liu, Catal. Lett. 150 (2020) 3553 (https://doi.org/10.1007/s10562-020-03253-5)
А. Ahmed Raza, S. Ravi, S. S. Tajudeen, A. K. Ibrahim Sheriff, React. Funct. Polym. 167 (2021) 105011 (https://doi.org/10.1016/j.reactfunctpolym.2021.105011)
D. Setamdideh, J. Serb. Chem. Soc. 81 (2016) 971 (https://doi.org/10.2298/JSC160202050S)
C. S. Marvel, T. H. Shepherd, J. Org. Chem. 24 (1959) 599 (https://doi.org/10.1021/jo01087a006)
а) P. Kulal, V. Badalamoole, Int. J. Biol. Macromol. 193 (2021) 2232 (https://doi.org/10.1016/j.ijbiomac.2019.11.181); b) H. Ge, Z. Zhang, X. Zhao, H. Li, J. Sun, X. Jv, Can. J. Chem. Eng. 99 (2021) S157 (https://doi.org/10.1002/cjce.24014)
a) G. Sharma, A. Kumar, M. Naushad, B. Thakur, D. V. N. Vo, B. Gao, A. A. Al-Kahtanid, F. J . Stadler, J. Hazard. Mater. 416 (2021) 125714 (https://doi.org/10.1016/j.jhazmat.2021.125714); b) S. Dan, S. Banivaheb, H. Hashemipour, M. kalantari, Polym. Bull. 78 (2021) 1887 (https://doi.org/10.1007/s00289-020-03190-8); c) T. Sharika, A. Mohanan, Mater. Today: Proc. 41 (2021) 744 (https://doi.org/10.1016/j.matpr.2020.08.421)
S. Bujok, M. Konefal, R. Konefal, M. Nevoralová, S. Bednarz, K. Mielczarek, H. Benes, J. Colloid Interface Sci. 610 (2022) 1 (https://doi.org/10.1016/j.jcis.2021.12.055)
C. Cottet, A. G. Salvay, M. A. Peltzer, M. Fernandez-Garcia, Polymers, 13 (2021) 200 (https://doi.org/10.3390/polym13020200)
L. Wang, G. Guan, J. Wang, Iran. Polym. J. 30 (2021) 1149 (https://doi.org/10.1007/s13726-021-00959-0)
G. Z. Yin, J. L. Palencia, D. Y. Wang, Compos. Commun. 27 (2021) 100893 (https://doi.org/10.1016/j.coco.2021.100893)
T. C. Sung, M. W. Lu, Z. Tian, H. H. C. Lee, J. Pan, Q. D. Ling, A. Higuchi. J. Mater. Chem. B. 9 (2021) 7662 (https://doi.org/10.1039/D1TB01555A)
H. Baskan, L. Esentürk, S. Dösler, A. S. Sarac, H. Karakas, J. Ind. Text. 50 (2019) 1594 (https://doi.org/10.1177/1528083719868170)
G. Zhu, H. Hub, T. Yang, J. Ma, H. Zhang, X. He, RSC Adv. 11 (2021) 20720 (https://doi.org/10.1039/D1RA03109K)
a) S. Esmaielzadeh, D. Setamdideh, J. Serb. Chem. Soc. 86 (2021) 1039 (https://doi.org/10.2298/JSC210521059E); b) P. Hamdi Mohamadabad, D. Setamdideh, Org. Prep. Proced. Int. 55 (2023) 265 (https://doi.org/10.1080/00304948.2022.2141044); c) S. Esmaielzadeh, D. Setamdideh, F. Ghanbary, J. Mex. Chem. Soc. 68 (2024) 234 (https://doi.org/10.29356/jmcs.v68i2.1910)
E. Grespos, D. J. T. Hill, J. H. O’Donnell, P. W. O’Sullivan, T. L. Young, J. C. East, K. J. Ivin, Makromol. Chem. Rapid Commun. 5 (1984) 489 (https://doi.org/10.1002/marc.1984.030050901)
J. Velickovic, J. Filipovic, D. P. Petrovic, Polym. Bull. 32 (1994) 169 (https://doi.org/10.1007/BF00306384)
M. Cao, Synthesis and properties of poly(itaconic acid). University of New Hampshire. England. 2008.
S. Bednarz, A. Błaszczyk, D. Błażejewska, D. Bogdał, Catal. Today 257 (2015) 297 (https://doi.org/10.1016/j.cattod.2014.07.021)
S. Bednarza, G. Kowalskib, R. Konefałc, Eur. Polym. J. 115 (2019) 30 (https://doi.org/10.1016/j.eurpolymj.2019.03.021)
R. Sarkar, C. Mukhopadhyay, Tetrahedron Lett. 54 (2013) 3706 (https://doi.org/10.1016/j.tetlet.2013.05.017)
R. Ghorbani-Vaghei, N. Sarmast, J. J. Mahmoodi, Appl. Organomet. Chem. 31 (2017) e3681 (https://doi.org/10.1002/aoc.3681)
J. Sun, Q. Wu, E. Y. Xia, C. G. Yan, Eur. J. Org. Chem. (2011) 2981 (https://doi.org/10.1002/ejoc.201100008)
A. M. Zonouz, I. Eskandari, B. Notash, Synth. Commun. 45 (2015) 2115 (https://doi.org/10.1080/00397911.2015.1065506)
H. Ahankar, A. Ramazani, K. Slepokura, T. Lis, S. W. Joo, Green Chem. 18 (2016) 3582 (https://doi.org/10.1039/c6gc00157b)
K. S. Marapala, N. Venkatesh, M. Swapna, P. R. Venkateswar, Int. J. ChemTech Res. 13 (2020) 227 (https://doi.org/10.20902/ijctr.2019.130128)
S. Pervaram, D. Ashok, C. Venkata Ramana Reddy, M. Sarasija, A. Ganesh, Chemical Data Collections, 29 (2020) 100508 (https://doi.org/10.1016/j.cdc.2020.100508)
N. Ghaffari Khaligh, T. Mihankhah, M. Rafie Johan, S. J. J. Titinchi, Green Process Synth. 8 (2019) 373 (https://doi.org/10.1515/gps-2019-0004)
N. Ghaffari Khaligh, T. Mihankhah, M. Rafie Johan, M. Synth. Commun. 49 (2019) 1334 (https://doi.org/10.1080/00397911.2019.1601225).