Comparative study of micellization and surface properties of cationic and anionic surfactants in acetonitrile–water mixed media Scientific paper
Main Article Content
Abstract
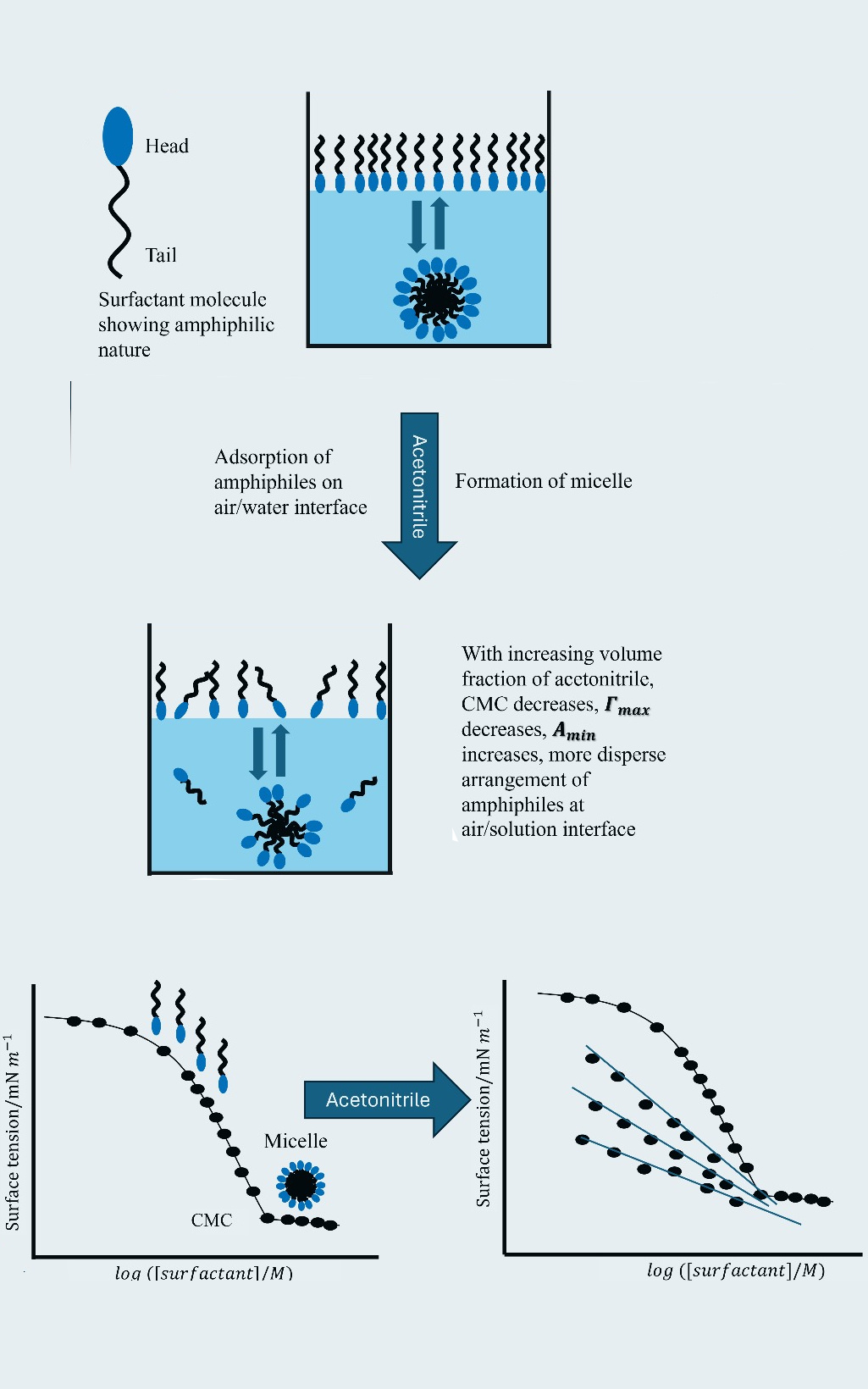
A comparative study was conducted to investigate the micellization behaviour, surface properties and wettability of the cationic surfactant cetyltrimethylammonium bromide (CTAB) and the anionic surfactant sodium dodecyl sulfate (SDS) in acetonitrile–water (ACN–water) mixed media. Surface tension and contact angle measurements were performed in pure water and ACN–water mixtures (0.10, 0.20 and 0.40 volume fractions of ACN) at 298.15 K to determine the critical micelle concentration (CMC), surface excess concentration (Γmax), minimum surface area per molecule (Amin), micellar surface pressure (πCMC) and packing parameter (P). Contact angle measurements were used to assess wettability on borosilicate glass surfaces. Results indicate that increasing ACN content leads to an increase in CMC, suggesting reduced micellization feasibility in less polar media. Surface excess concentration decreases with higher ACN fractions, while minimum surface area per molecule increases, indicating looser molecular packing at the air/solution interface. Contact angle measurements reveal a decrease in wettability with higher ACN content, demonstrating enhanced surfactant adsorption at the solid-liquid interface. Additionally, micellar surface pressure and packing parameter decline with increasing ACN concentration. These findings underscore the critical role of solvent composition in modifying surfactant aggregation and interfacial behaviour.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
M. J. Rosen, J. T. Kunjappu, Surfactants and Interfacial Phenomena, 4th ed., Wiley, New York, 2012, pp. 1–33 (https://doi.org/10.1002/0471670561)
M. E. Jiménez-Castañeda, D. I. Medina, Water 9 (2017) 235 (https://doi.org/10.3390/w9040235)
M. X. Gao, C. F. Liu, Z. L. Wu, Q. L. Zeng, X. X. Yang, W. B. Wu, Y. F. Li, C. Z. Huang, Chem. Comm. 49 (2013) 8015 (https://doi.org/10.1039/C3CC44624G)
T. F. Tadros, An Introduction to Surfactants, De Gruyter, Berlin, 2014, pp. 179–220 (https://doi.org/10.1515/9783110312133.179)
T. Wang, D. Chang, D. Huang, Z. Liu, Y. Wu, H. Liu, H. Yuan, Y. Jiang, Appl. Microbiol. Biotechnol. 105 (2021) 7619 (https://doi.org/10.1007/s00253-021-11602-6)
I. Kralova, J. Sjöblom, J. Dispers. Sci. Technol. 30 (2009) 1363 (https://doi.org/10.1080/01932690902735561)
S. K. Shah, A. Bhattarai, J. Chem. 2020 (2020) 4653092 (https://doi.org/10.1155/2020/4653092)
H. A. Bhuiyan, J. M. Khan, M. R. Islam, S. Rana, A. Ahmad, M. A. Hoque, M. M. Rahman, S. E. Kabir, Int. J. Biol. Macromol. 222 (2022) 181 (https://doi.org/10.1016/j.ijbiomac.2022.09.169)
S. K. Shah, S. K. Chatterjee, A. Bhattarai, J. Chem. 2016 (2016) 2176769 (https://doi.org/10.1155/2016/2176769)
S. K. Shah, S. K. Chatterjee, A. Bhattarai, J. Surfactants Deterg. 19 (2016) 201 (https://doi.org/10.1007/s11743-015-1755-x)
Y. Ghimire, S. Amatya, S. K. Shah, A. Bhattarai, SN. Appl. Sci. 2 (2020) 1295 (https://doi.org/10.1007/s42452-020-3036-1)
L. G. Ionescu, T. Tokuhiro, B. J. Czerniawski, E. S. Smith, in Solution Chemistry of Surfactants, K. L. Mittal, Ed., Plenum Press, New York, 1979, p. 487 (https://doi.org/10.1007/978-1-4615-7880-2_24)
T. P. Niraula, S. K. Shah, S. K. Chatterjee, A. Bhattarai, Karbala Int. J. Mod. Sci. 4 (2018) 26 (https://doi.org/10.1016/j.kijoms.2017.10.004)
M. Bielawska, A. Chodzińska, B. Jańczuk, A. Zdziennicka, Colloids Surfaces, A 424 (2013) 81 (https://doi.org/10.1016/j.colsurfa.2013.02.017)
S. Das, S. Mondal, S. Ghosh, J. Chem. Eng. Data 58 (2013) 2586 (https://doi.org/10.1021/je4004788)
P. K. Misra, B. K. Mishra, G. B. Behera, Colloids Surfaces 57 (1991) 1 (https://doi.org/10.1016/0166-6622(91)80175-N)
J. Šteflová, M. Štefl, S. Walz, M. Knop, O. Trapp, Electrophoresis 37 (2016) 1287 (https://doi.org/10.1002/elps.201500553)
F. Jalali, A. Gerandaneh, J. Dispers. Sci. Technol. 32 (2011) 659 (https://doi.org/10.1080/01932691003800049)
A. J. Ležaić, N. Pejić, J. Goronja, L. Pavun, D. Ðikanović, A. Malenović, Maced. J. Chem. Chem. Eng. 40 (2021) 277 (https://doi.org/10.20450/mjcce.2021.2394)
R. K. Banjare, M. K. Banjare, S. Panda, J. Solution Chem. 49 (2020) 34 (https://doi.org/10.1007/s10953-019-00937-4)
S. K. Shah, A. Giri, S. Adhikari, A. Bhattarai, Tenside Surfactants, Detergents 62 (2025) 132 (https://doi.org/10.1515/tsd-2024-2642)
N. R. Biswal, S. Paria, Ind. Eng. Chem. Res. 51 (2012) 10172 (https://doi.org/10.1021/ie301198k)
S. K. Shah, P. K. Das, A. Bhattarai, Heliyon 11 (2025) e42352 (https://doi.org/10.1016/j.heliyon.2025.e42352)
S. K. Shah, R. M. Leblanc, A. Bhattarai, Results Chem. 15 (2025) 102262 (https://doi.org/10.1016/j.rechem.2025.102262)
K. M. Sachin, S. A. Karpe, M. Singh, A. Bhattarai, Heliyon 5 (2019) e01510 (https://doi.org/10.1016/j.heliyon.2019.e01510)
R. A. Khalil, A. H. A. Zarari, Appl. Surf. Sci. 318 (2014) 85 (https://doi.org/10.1016/j.apsusc.2014.01.046)
C. Tanford, The hydrophobic effect: formation of micelles and biological membranes, J. Wiley & Sons, 1980
D. Das, K. Ismail, J. Coll. Interface Sci. 327 (2008) 198 (https://doi.org/10.1016/j.jcis.2008.07.045)
E. H. Lucassen-Reynders, J. Phys. Chem. 67 (1963) 969 (https://doi.org/10.1021/j100799a005).