Removal of nickel(II) ions during water purification with ferrous sulfate. Part 2. Structure and composition of iron(III) hydroxide precipitates Scientific paper
Main Article Content
Abstract
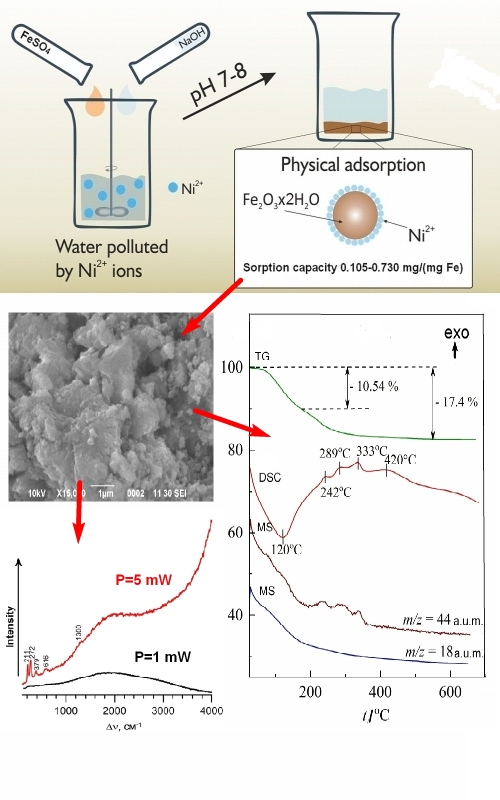
A comparative analysis of the composition and structure of freshly precipitated iron(III) hydroxide precipitates obtained from a solution of iron(II) sulfate in the presence of sodium sulfate (400 mg L-1) at pH 7 and 8, before and after the sorption of nickel ions onto them, was carried out. Using IR and Raman spectroscopy, X-ray phase and thermogravimetric analysis, it was shown that the precipitates have the general (gross) formula Fe2O3×2H2O and contain small amounts of goethite (α-FeOOH) and lepidocrocite (γ-FeOOH). It has been established that the sorption of nickel ions onto these precipitates is not accompanied by chemisorption, i.e., no mixed compounds between iron and nickel are formed. The point of zero charge of the precipitate particles is at pH 5.4, with a positive zeta potential below and a negative zeta potential above this pH. The introduction of nickel ions into the solution leads to the appearance of a second zero charge point at pH 10.2.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Russian Foundation for Fundamental Investigations
Grant numbers 20-48-660038 -
Ministry of Science and Higher Education of the Russian Federation
Grant numbers 124020600024-5
References
O. D. Linnikov, I. V. Rodina, J. Serb. Chem. Soc. 91 (2026) 39 (https://doi.org/10.2298/JSC250407080L)
M. Kiyama, T. Takada, Bull. Chem. Soc. Japan 45 (1972) 1923
T. Misawa, K. Yashimoto, S. Shimodaira, Corros. Sci. 14 (1974) 131
Y. Deng, Water Res. 31 (1997) 1347 (https://doi.org/10.1016/s0043-1354(96)00388-0)
R. R. Kleshcheva, D. A. Zherebtsov, V. Sh. Mirasov, D. G. Kleshchev, Bull. South Ural State Univ. 1 (2012) 17
E. V. Petrova, A. F. Dresvyannikov, M. A. Tsyganova, A. M. Gubaidullina, D. V. Wasserman, N. I. Naumkina, Bull. Kazan Technol. Univ. 2 (2009) 24
O. D. Linnikov, I. V. Rodina, G. S. Zakharova, K. N. Mikhalev, I. V. Baklanova, Yu. V. Kuznetsova, A. Yu. Germov, B. Yu. Goloborodskii, A. P. Tyutyunnik, Z. A. Fattakhova, Water Environ. Res. 94 (2022) e10827 (https://doi.org/10.1002/wer.10827)
M. Hanesch, Geophys. J. Int. 177 (2009) 941 (https://doi.org/10.1111/j.1365-246X.2009.04122.x)
D. L. de Faria, S. S. Venâncio, M. T. de Oliveira, J. Raman Spectrosc. 28 (1997) 873 (https://doi.org/10.1002/(SICI)1097-4555(199711)28:11<873::AID-JRS177>3.0.CO;2-B)
M. A. Legodi, D. de Waal, Dyes Pigments 74 (2007) 161 (https://doi.org/10.1016/j.dyepig.2006.01.038)
M. Kosmulski, S. Durand-Vidal, E. Mazcka, J. B. Rosenholm, J. Col. Interface Sci. 271 (2004) 261 (https://doi.org/10.1016/j.jcis.2003.10.032)
E. Paterson, R. Swaffield, J. Therm. Anal. 18 (1980) 161 (https://doi.org/10.1007/bf01909464)
M. V. Akhmanova, G. I. Malofeeva, N. P. Andreeva, J. Anal. Chem. 31 (1976) 447
L. G. Berg, K. P. Pribylov, V. P. Egunov, R. A. Abdurakhmanov, Russ. J. Inorg. Chem. 14 (1969) 2303
J. Liu, R. Zhu, L. Ma, H. Fu, X. Lin, S. C. Parker, M. Molinari, Geoderma 383 (2021) 114799 (https://doi.org/10.1016/j.geoderma.2020.114799)
M. Villalobos, J. Antelo, Rev. Intern. Contam. Amb. 27 (2011) 139 (https://doi.org/10.20937/RICA.25013)
M. A. Inam, R. Khan, K-H. Lee, Y-M. Wie, Int. J. Environ. Res. Pub. Health 18 (2021) 9812 (https://doi.org/10.3390/ijerph18189812).