Green route for efficient synthesis of metal complexes of 4-bromo-2-((E)-((2-hydroxyphenyl)imino)methyl)-6-((E)-(3- -nitrophenyl)diazenyl)phenol and its anti-hyperglycemia, anticancer and antimicrobial assessment Scientific paper
Main Article Content
Abstract
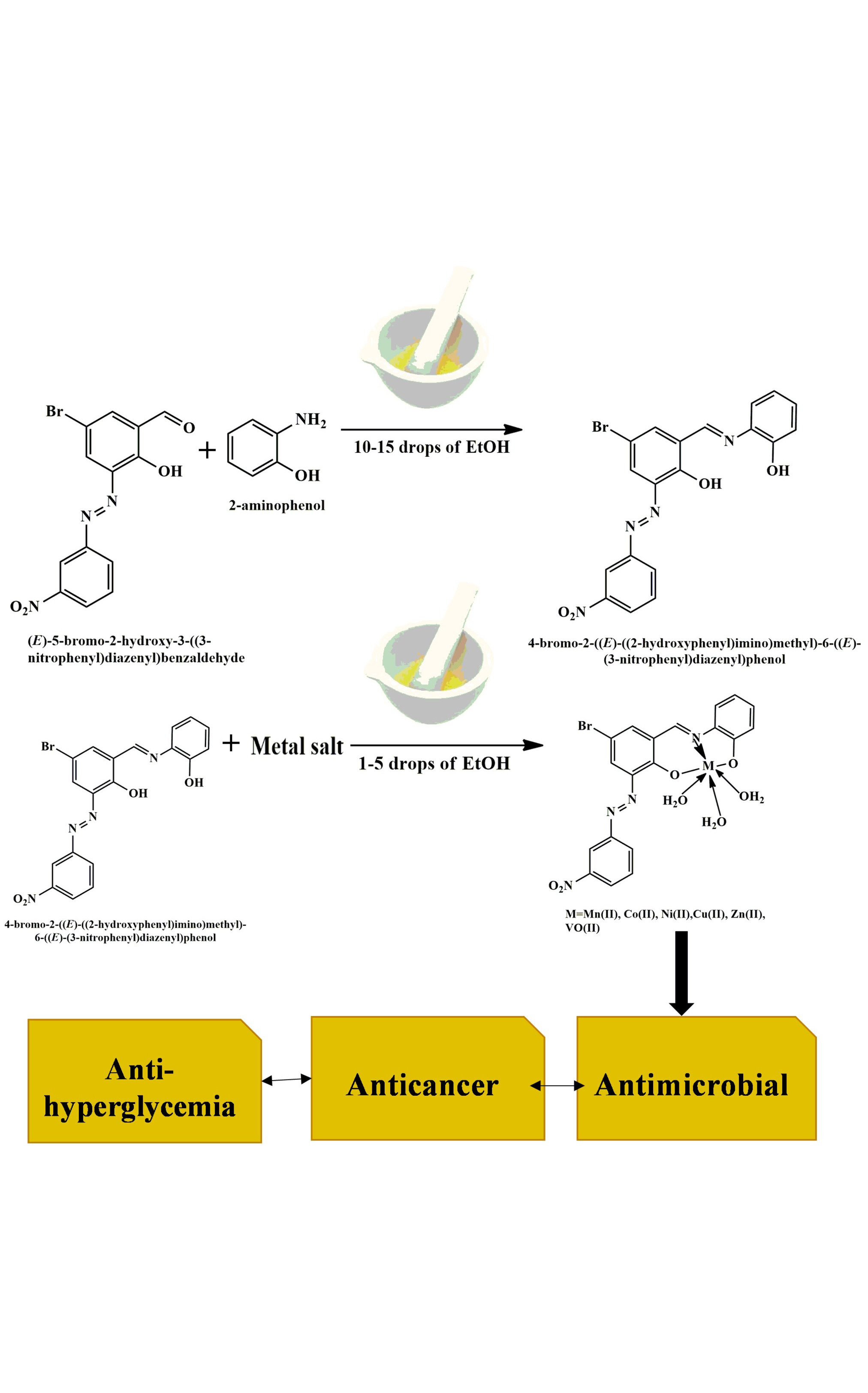
The world is battling cancer and diabetes, prompting global research into effective drugs. Studies show coordination compounds, especially substituted salicylaldehydes, exhibit strong biological activity, which increases when treated with amines. In the presented article, the preparation of metal complexes involving Mn(II), Co(II), Ni(II), Cu(II), Zn(II) and VO(II) was carried out using a Schiff base ligand that had been previously synthesized by grinding method of (E)-5-bromo-2-hydroxy-3-((3-nitrophenyl)diazenyl)benzaldehyde and 2-aminophenol. The synthesized Schiff base ligand confirmed by mass spectra, 1H-NMR and FT-IR spectra. After the confirmation of the Schiff base ligand, synthesis of metal complexes by using metal salt of Mn(II), Co(II), Ni(II), Cu(II), Zn(II) and VO(II). The synthesized metal complexes were analysed by elemental analysis, FT-IR spectra, electronic spectra, thermal analysis, X-ray powder diffraction, molar conductivity, etc. For the biological studies, anti-hyperglycaemia and anticancer assessments of Schiff base ligand and metal complexes by alpha amylase inhibition assay and MTT assay against standard reference drug acarbose and 5-flourouracil (5-FU), respectively. The findings of the anti-hyperglycaemia suggest that Co(II) shows higher activity than other metals as well as all metal complexes shows significant activity than free ligand. In the anticancer activity it is clear that Co(II) shows higher activity than other meal complexes as well as all metal complexes shows higher activity than that of free ligand. In addition to this, the antimicrobial properties were examined against two Gram-positive bacterial strains (Staphylococcus aureus and Bacillus subtilis), two Gram-negative bacterial strains (Klebsiella pneumoniae and Pseudomonas aeruginosa) and three fungal strains (Penicillium chrysogenum, Trichoderma viride and Aspergillus niger). From all the result and observations, it is clear that metal complexes exhibit higher biological assessments than that of Schiff base ligand.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
E. S. Aazam, R. Thomas, J. Mol. Struct. 1295 (2024) 136669 (https://doi.org/10.1016/j.molstruc.2023.136669)
R. Gandhimathi, S. Anbuselvi, R. Saranya, J. Indian Chem. Soc. 100 (2023) 101033 (https://doi.org/10.1016/j.jics.2023.101033)
S. Sharma, S. P. Yadav, S. Kumari, M. Ranka, J. Indian Chem. Soc. 100 (2023) 100997 (https://doi.org/10.1016/j.jics.2023.100997)
H. L. Singh, S. Khaturia, V. S. Solanki, N. Sharma, J. Indian Chem. Soc. 100 (2023) 100945 (https://doi.org/10.1016/j.jics.2023.100945)
P. Tiwari, S. Phadte, S. Chandavarkar, B. Biradar, S. M. Desai, J. Indian Chem. Soc. 100 (2023) 100951 (https://doi.org/10.1016/j.jics.2023.100951)
M. R. A. Mohameed, A. H. Hasan, R. D. Kareem, J. Garmian Univ. 155 ( 2017) 453 (https://doi.org/10.24271/garmian.155)
H. Katouah, A. M. Hameed, A. Alharbi, F. Alkhatib, R. Shah, S. Alzahrani, R. Zaky, N. M. El-Metwaly, Chem. Select 5 (2020) 10256 (https://doi.org/10.1002/slct.202002388)
G. More, S. Bootwala, S. Shenoy, J. Mascarenhas, K. Aruna, Int. J. Pharm. Sci. 9 (2018) 3029 (http://dx.doi.org/10.13040/IJPSR.0975-8232.9(7).3029-35)
K. N. Sarwade, K. B. Sakhare, M. A. Sakhare, S. V. Thakur, Heterocyclic Lett. 14 (2024) 797 (https://www.heteroletters.org/issue144/Paper-8.pdf)
B. Debaraj, P. Raj, R. Badekar, K. I. Momin, A. S. S. Bondge, J. Appl. Organomet. Chem. 4 (2024) 76 (https://doi.org/10.48309/JAOC.2024.434283.1156)
V. P. Radha and M. Prabakaran, Appl. Organomet. Chem. 36 (2022) e6872 (https://doi.org/10.1002/aoc.6872)
S. A. Halim, M. Shebl, J. Coord. Chem. 74 (2021) 2984 (https://doi.org/10.1080/00958972.2021.2020259)
M. Shebl, J. Coord. Chem. 69 (2016) 199 (https://doi.org/10.1080/00958972.2015.1116688)
O. M. I. Adly, M. Shebl, E. M. Abdelrhman, B. A. El-Shetary, J. Mol. Struct. 1219 (2020) 128607 (https://doi.org/10.1016/j.molstruc.2020.128607)
K. Tahmineh, H. Mohammad, H. Hassan, A. H. Hasan, Chem. Methodol. 7 (2023) 748 (https://doi.org/10.48309/chemm.2023.414603.1718)
C. Dolan, R. Glynn, S. Griffin, C. Conroy, C. Loftus, Diabet. Med. 35 (2018) 871 (https://doi.org/10.1111/dme.13639)
R. Miyazaki, H. Yasui, Y. Yoshikawa, Open J. Inorg. Chem. 6 (2016) 114 (https://doi.org/10.4236/ojic.2016.62007)
E. Akila, M. Usharani, P. Maheswaran, R. Rajavel, Int. J. Rec. Sci. Res. 4 (2013) 1497 (https://www.recentscientific.com/sites/default/files/Download_629.pdf)
C. Veeravel, K. Rajasekar, P. Chakkaravarthy, R. Selvarani, A. Kosiha, V. Sathya, Research Square (2023) 2693 (https://doi.org/10.21203/rs.3.rs-2680647/v1)
P. Wanjari, A. Bharati, V. Ingle, Malaysian J. Chem. 23 (2021) 23 (https://ikm.org.my/publications/malaysian-journal-of-chemistry/view-abstract.php?abs=J0035-C0R305)
L. M. R. Ummi, K. Karima, M. T. Amalina, K. Y. Muhamad, N. A. Zakaria, Malaysian J. Chem. 24 (2022) 250 (https://doi.org/10.55373/mjchem.v24i2.250)
V. P. Nisha, N. Subhadrambika, S. S. Swathy, K. Mohanan, J. Indian Chem. Soc. 89(2012) 761 (https://www.researchgate.net/publication/288108395_Synthesis_spectroscopic_characterization_thermal_decomposition_and_antimicrobial_studies_of_manganeseIII_ironIII_and_cobaltIII_complexes_with_Schiff_base_derived_from_thiophene-2-carboxaldehyde_and_2-)
M. Bal, G. Ceyhan, B. Avar, M. Kose, A. Kayraldız, M. Kurtoglu, Turkish J. Chem. 38 (2014) 222 (https://doi.org/10.3906/kim-1306-28)
M. Kurtoglu, E. Ispir, N. Kurtoglu, S. Serin, Dyes Pigments 77 (2008) 75 (https://doi.org/10.1016/j.dyepig.2007.03.010)
F. Dimiza, A. N. Papadopoulos, V. Tangoulis, V. Psycharis, C. P. Raptopoulou, D. P. Kessissoglou, G. Psomas, Dalton Transact. 39 (2010) 4517 (https://doi.org/10.1039/B927472C)
M. M. Abd-Elzaher, S. A. Moustafa, A. A. Labib, M. M. Ali, Monatsh. Chem. 141 (2010) 387 (https://doi.org/10.1007/s00706-010-0268-6)
E. Ispir, Dyes Pigments 82 (2009) 13 (https://doi.org/10.1016/j.dyepig.2008.09.019)
M. Ozdemir, Inorg. Chim. Acta 421 (2014) 1 (https://doi.org/10.1016/j.ica.2014.05.024)
S. Urus, M. Dolaz, M. Tumer, J. Inorg. Org. Poly. Mat. 20 (2010) 706 (http://dx.doi.org/10.1007/s10904-010-9394-1)
V. Anusuya, N. Muruganantham, P. Anitha, S. Mahesh, Oriental J. Chem. 38 (2022) 1525 (https://doi.org/10.13005/ojc/380626)
K. N. Sarwade, K. B. Sakhare, M. A. Sakhare, S. V. Thakur, Eurasian J. Chem. 30 (2025) 4 (https://doi.org/10.31489/2959-0663/1-25-8)
K. Singh, Y. Kumar, P. Puri, C. Sharma, K. R. Aneja, Arabian J. Chem. 10 (2017) 978 (http://dx.doi.org/10.1016/j.arabjc.2012.12.038)
Z. H. Abd El-Waheb, M. M. Mashaly, A. A. Faheim, Chem. Papers 59 (2005) 25 (https://www.chemicalpapers.com/?id=7&paper=55)
Y. N. Bharate, K. B. Sakhare, S. A. Survase, M. A. Sakhare, Heterocyclic Lett. 13 (2023) 45 (https://www.heteroletters.org/issue131/Paper-5.pdf)
K. N. Sarwade, K. B. Sakhare, M. A. Sakhare, S. V. Thakur, Mong. J. Chem. 25 (2024) 10 (https://doi.org/10.5564/mjc.v25i52.3537)
R. Sawant, J. Wadekar, R. Ukirde, G. Barkade, Pharmaceut. Sci. 27 (2021) 345 (http://dx.doi.org/10.34172/PS.2020.95)
G. Sasikumar, T. N. Balaji, A. K. Ibrahim Sheriff, World J. Pharm. Res. 7 (2018) 564 (https://www.wjpr.net/abstract_file/9977)
H. R. Afzal, N. U. H. Khan, K. Sultana, A. Mobashar, A. Lareb, A. Khan, A. Gull, H. Afzaal, M. T. Khan, M. Rizwan, M. Imran, ACS Omega 6 (2021) 4470 (https://doi.org/10.1021/acsomega.0c06064)
L. H. Abdel-Rahman, A. M. Abu-Dief, R. M. El-Khatib, S. M. Abdel-Fatah, Bioorg. Chem. 69 (2016) 140 (https://doi.org/10.1016/j.bioorg.2016.10.009)
K. N. Sarwade, K. B. Sakhare, M. A. Sakhare, Y. N. Bharate, S. V. Thakur, Curr. Bioact. Comp. (2025) e15734072349875 (https://doi.org/10.2174/0115734072349875250224092826).