Divergent synthesis and antitumour activity of novel conformationally constrained (–)-muricatacin analogues Scientific paper
Main Article Content
Abstract
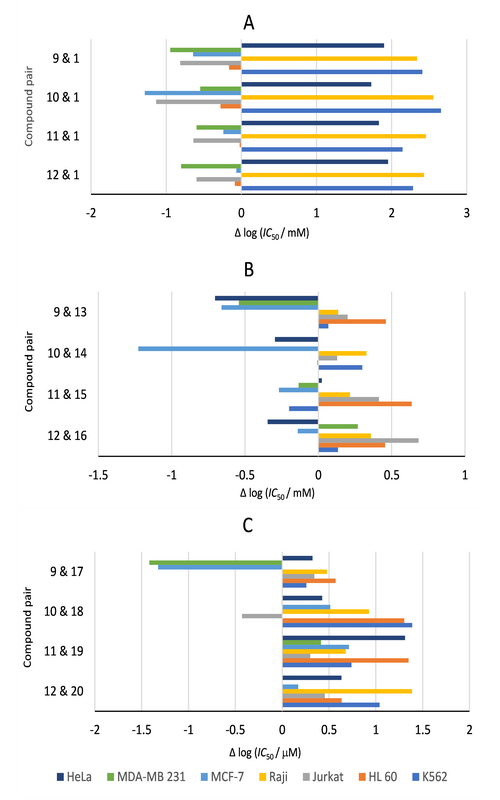
Four novel conformationally restricted (–)-muricatacin analogues, bearing a methoxy group at the C-5 position and with an alkoxymethyl group аs the C-7 side chain, have been synthesised and their in vitro antiproliferative activity was evaluated against a panel of seven human tumour cell lines, as well as a single normal cell line. All analogues (9–12) showed diverse antiproliferative effects against all tested human malignant cell lines, but were devoid of any significant cytotoxicity towards the normal foetal lung fibroblasts (MRC-5). A structure–activity relationship study reveals that the introduction of tetrahydrofuran ring, the replacement of C-8 methylene group in the side chain of muricatacin analogues with the O-8 ether functionality, as well as the length of side chain may be beneficial for the antiproliferative effects of these lactones. All novel analogues were more potent than lead compound, (–)-muricatacin, against HL-60 cell line.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-68/2020-14/200125 -
Serbian Academy of Sciences and Arts
Grant numbers 01-2019-F65 -
Serbian Academy of Sciences and Arts
Grant numbers F-130
References
M. J. Rieser, J. F. Kozlowski, K. V. Wood, J. L. McLaughlin, Tetrahedron Lett. 32 (1991) 1137 (https://doi.org/10.1016/S0040-4039(00)92027-6)
C.-C. Liaw, F.-R. Chang, S.-L. Chen, C.-C. Wu, K.-H. Lee, Y. C. Wu, Bioorg. Med. Chem. 13 (2005) 4767 (https://doi.org/10.1016/j.bmc.2005.05.008)
A. Cavé, C. Chaboche, B. Figadère, J. C. Harmange, A. Laurens, J. F. Peyrat, M. Pichon, M. Szlosek, J. Cotte-Lafitte, A. M. Quéro, Eur. J. Med. Chem. 32 (1997) 617 (https://doi.org/10.1016/S0223-5234(97)83287-4)
M. Chandrasekhar, K. L. Chandra, V. K. Singh, ARKIVOC VII (2002) 34 (https://doi.org/10.3998/ark.5550190.0003.705)
K. J. Quinn, A. K. Isaacs, R. A. Arvary, Org. Lett. 6 (2004) 4143 (https://doi.org/10.1021/ol040047f)
B. Dhotare, A. Chattopadhyay, Tetrahedron Lett. 46 (2005) 3103 (https://doi.org/10.1016/j.tetlet.2005.03.002)
V. Popsavin, B. Srećo, G. Benedeković, M. Popsavin, J. Francuz, V. Kojić, G. Bogdanović, Bioorg. Med. Chem. Lett. 18 (2008) 5182 (https://doi.org/10.1016/j.bmcl.2008.08.097)
M. T. Barros, M. A. J. Charmier, C. D. Maycock, T. Michaud, Tetrahedron 65 (2009) 396 (https://doi.org/10.1016/j.tet.2008.10.020)
M. González, Z. Gándara, B. Covelo, G. Gómez, Y. Fall, Tetrahedron Lett. 52 (2011) 5983 (https://doi.org/10.1016/j.tetlet.2011.08.160)
Y.-F. Tsai, C.-C. Huang, S.-H. Lin, P.-M. Su, Y.-J. Chen, T.-Y. Wu, Heterocycles 85 (2012) 299 (https://doi.org/10.3987/COM-11-12397)
M. González, Z. Gándara, Z. Pazos, G. Gómez, Y. Fall, Synthesis (2013) 625 (https://doi.org/10.1055/s-0032-1318113)
S. Chatterjee, A. Manna, T. Bhaumik, Tetrahedron: Asymmetry 25 (2014) 1624 (https://doi.org/10.1016/j.tetasy.2014.11.001)
D. A. Chaudhari, A. B. Ingle, R. A. Fernandes, Tetrahedron: Asymmetry 27 (2016) 114 (https://doi.org/10.1016/j.tetasy.2016.01.003)
S. Yaragorla, R. Muthyala, ARKIVOC (2010) 178 (https://doi.org/10.3998/ark.5550190.0011.a15)
C. R. Reddy, D. Suman, N. N. Rao, Helv. Chim. Acta 98 (2015) 967 (https://doi.org/10.1002/hlca.201400356)
C. Cooze, A. Manchoju, S. V. Pansare, Synlett (2017) 2928 (https://doi.org/10.1055/s-0036-1590858)
S. H. Tsai, P. C. Hsieh, L. L. Wei, H. F. Chiu, Y. C. Wu, M. J. Wu, Tetrahedron Lett. 40 (1999) 1975 (https://doi.org/10.1002/chin.199923295)
J. M. Andres, N. de Elena, R. Pedrosa, A. Pérez-Encabo, Tetrahedron: Asymmetry 12 (2001) 1503 (https://doi.org/10.1016/S0957-4166(01)00044-1)
V. Popsavin, I. Krstić, M. Popsavin, B. Srećo, G. Benedeković, V. Kojić, G. Bogdanović Tetrahedron 62 (2006) 11044 (https://doi.org/10.1016/j.tet.2006.09.054)
B. Srećo, G. Benedeković, M. Popsavin, P. Hadžić, V. Kojić, G. Bogdanović, V. Divjaković, V. Popsavin, Tetrahedron 67 (2011) 9358 (https://doi.org/10.1016/j.tet.2011.09.132)
B. Srećo Zelenović, S. Kekezović, M. Popsavin, V. Kojić, G. Benedeković, V. Popsavin, J. Serb. Chem. Soc. 84 (2019) 1345 (https://doi.org/10.2298/JSC190912104S)
D. A. Scudiero, R. H. Shoemaker, K. D. Paull, A. Monks, S. Tierney, T. H. Nofziger, M. J. Currens, D. Seniff, M. R. Boyd, Cancer. Res. 48 (1988) 4827 (https://pdfs.semanticscholar.org/3299/2997d7d34c82c2ce34937b25c5a770dbd735.pdf)
J. Francuz, M. Popsavin, S. Djokić, V. Kojić, T. Srdić-Rajić, M. Rodić, D. Jakimov, V. Popsavin, Med. Chem. Commun. 9 (2018) 2017 (https://doi.org/10.1039/C8MD00431E)
D. Cremer, J. A. Pople, J. Am. Chem. Soc. 97 (1975) 1354 (https://dx.doi.org/10.1021/ja00839a011)
D. Cremer, Isr. J. Chem. 20 (1980) 12 (https://dx.doi.org/https://doi.org/10.1002/ijch.198000048).