Encapsulation of peach waste extract in Saccharomyces cerevisiae cells Scientific paper
Main Article Content
Abstract
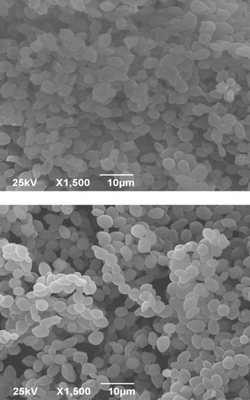
As a secondary industry product, peach waste (PW) presents an ecological problem, but a potentially rich source of natural antioxidants. A potential and novel way to improve the phytochemical stability of waste rich in phytochemicals is encapsulation in yeast cells which possess good structure characteristics. In the present study, PW extract was encapsulated in non-plasmolyzed, plasmolyzed, and living Saccharomyces cerevisiae cells by freeze-drying method. HPLC analysis revealed that β-carotene is the most abundant carotenoid, while epicatechin and catechin are the most abundant phenolics in PW. The highest encapsulation efficiency of carotenoids (86.59 %), as well as phenolics (66.98 %), was obtained with freeze-dried non-plasmolyzed yeast cells used as carriers. Although plasmolysis can cause some changes in yeast cell structure and properties, it did not enhance the encapsulation efficiency of present phytochemicals. Successful encapsulation of PW extract in yeast cells was confirmed by FTIR spectroscopy and SEM imaging. The obtained results present the encapsulation of sensitive compounds in yeast cells by freeze-drying as an excellent method for preserving valuable compounds and their potential use in the food and pharmaceutical industry.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
E. I. Paramera, S. J. Konteles, V. T. Karathanos, Food Chem., A 74 (2011) 125 (https://doi.org/10.1016/j.foodchem.2010.09.063)
B. N. Pham-Hoang, C. Romero-Guido, Y. Waché, Appl. Microbiol. Biotechnol. 97 (2013) 6635 (https://doi.org/10.1007/s00253-013-5044-1)
E. I. Paramera, S. J. Konteles, V. T. Karathanos, Food Chem., B 125 (2011) 913 (https://doi.org/10.1016/j.foodchem.2010.09.071)
R. Salari, O. Rajabi, Z. Khashyarmanesh, M. Fathi Najafi, B. S. FazlyBazzaz Iranian J. Pharm. Res. 14 (2015) 1247 (PMCID: PMC4673954)
S. Mokhtari, S. Mahdi Jafari, M. Khomeiri, Y. Maghsoudlou, Y. Ghorbani, Food Res. Int. 96 (2017) 1 (https://doi.org/10.1016/j.foodres.2017.03.014)
G. Shi, L. Rao, H. Yu, H. Xiang, G. Pen, S. Long, C. Yang, J. Food Eng. 80 (2007) 1060 (https://doi.org/10.1016/j.jfoodeng.2006.06.038)
C. K. Chow, S. Palecek, Biotech. Prog. 20 (2004) 449 (https://doi.org/10.1021/bp034216r)
V. Normand, G. Dardelle, P. E. Bouquerand, L. Nicolas, D. J. Johnston, J. Agric. Food Chem. 53 (2005) 7532 (https://doi.org/10.1021/jf0507893)
E. I. Paramera, V. T. Karathanos, S. J. Konteles, Yeast cells and yeast-based materials for microencapsulation, Elsevier Inc., Amsterdam, 2014, p. 267 (https://doi.org/10.1016/C2012-0-00852-6)
J. R. P. Bishop, G. Nelson, J. Lamb, J. Microencapsulation 15 (1998) 761 (https://doi.org/10.3109/02652049809008259)
G. Shi, L. Rao, H. Yu, H. Xiang, H. Yang, R. Ji, Int. J. Pharm. 349 (2008) 83 (https://doi.org/10.1016/j.ijpharm.2007.07.044)
F. Saidani, R. Giménez, C. Aubert, G. Chalot, J. A. Betrán, Y. Gogorcena, J. Food Compos. Anal. 62 (2017) 1 (https://doi.10.1016/j.jfca.2017.04.015)
I. Hasbay Adil, I. H. Çetin, M. E. Yener, A. Bayındırlı, J. Supercrit. Fluids 43 (2007) 55 (https://doi.org/10.1016/j.supflu.2007.04.012)
X. Liao, P. Greenspan, R. B. Pegg, Food Chem. 271 (2019) 1 (https://doi.org/10.1016/j.foodchem.2018.07.163)
A. Dalla Valle, I. Mignani, A. Spinardi, F. Galvano, S. Ciappellano, Eur. Food Res. Technol. 225 (2007) 167 (https://doi.org/10.1007/s00217-006-0396-8)
A. Schieber, F. C. Stintzing, R. Carle, Trends Food Sci. Technol. 12 (2001) 401 (https://doi.org/10.1016/S0924-2244(02)00012-2)
H. Kowalska, K. Czajkowska, J. Cichowska, A. Lenart, Trends Food Sci. Technol. 67 (2017) 1 (https://doi.org/10.1016/j.tifs.2017.06.016)
I. Hasbay Adil, H. I. Çetin, M. E. Yener, A. Bayındırli, J. Supercrit. Fluids 43 (2007) 55 (https://doi.org/10.1016/j.supflu.2007.04.012)
S. Rodríguez-González, I. F. Pérez-Ramírez, D. M. Amaya-Cruz, M. A. Gallegos-Cor¬ona, M. Ramos-Gomez, O. Mora, R. Reynoso-Camacho, J. Funct. Foods 45 (2018) 58 (https://doi.org/10.1016/j.jff.2018.03.010)
V. Šeregelj, G. Ćetković, J. Čanadanović-Brunet, V. Tumbas-Šaponjac, J. Vulić, S. Stajčić, Acta Period. Tech. 48 (2017) 261 (https://doi.org/10.2298/APT1748261S)
M. Nagata, I. Yamashita, Japan Soc. Food Sci. Technol. 39 (1992) 925 (https://doi.org/10.3136/nskkk1962.39.925)
A. Girones-Vilaplana, P. Mena, D. A. Moreno, C. Garcia-Viguera, J. Sci. Food Agric. 94 (2014) 1090 (https://doi.org/10.1002/jsfa.6370)
M. Oyaizu, Japanese J. Nutr. Dietet. 94 (1986) 307 (https://doi.org/10.5264/eiyogakuzashi.44.307)
M. S. Al-Saikhan, L. R. Howard, Jr. JC. Miller, J. Food Sci. 60 (1995) 341 (https://doi.org/10.1111/j.1365-2621.1995.tb05668.x)
V. Tumbas Šaponjac, G. Ćetković, J. Čanadanović-Brunet, B. Pajin, S. Djilas, J. Petrović, J. Vulić, Food Chem. 207 (2016) 27 (https://doi.org/10.1016/j.foodchem.2016.03.082)
D. Giuffrida, G. Torre, G. Dugo, Fruits 68 (2012) 39 (https://doi.org/10.1051/fruits/2012049)
B. T. Stojanovic, S. S. Mitic, G. S. Stojanovic, M. M. Mitic, D. A. Kostic, D. D. Paunovic, B. Arsic, Notulae Botanicae Horti Agrobotanici Cluj-Napoca 44 (2016) 175 (https://doi.org/10.15835/nbha44110192)
C. Andreotti, D. Ravaglia, A. Ragaini, G. Costa, Ann. App. Biol. 153 (1998) 11 (https://doi.org/10.1111/j.1744-7348.2008.00234.x)
C. Forni, F. Facchiano, M. Bartoli, S. Pieretti , A. Facchano, D. D'Arcamgelo, S. Norelli, G. Valle , R. Nisini , S. Beninati , C. Tabolacci, R. N. Jadeja, Bio. Med. Res. Int. (2019) (ID: 8748253)
E. Dadkhodazade, A. Mohammadi, S. Shojaee-Aliabadi, A. M. Mortazavian, L. Mirmoghtadae, S. M. Hosseni, Food Biophys. 13 (2018) 231 (https://doi.org/10.1007/s11483-018-9546-3)
M. Kavosi, A. Mohammadi, S. Shojaee-Aliabadi, R. Khaksar, S. M. Hosseini, J. Sci. Food Agric. 98 (2018) 1 (https://doi.org/10.1002/jsfa.8696)
G. J. Morris, L. Winters, G. E. Coulson, K. J. Clarke, J. Gen. Microbiol. 129 (1984) 2023 (https://doi.org/10.1099/00221287-132-7-2023).