Synthesis, characterization and biological activity of Pt(II) complexes with steroidal thiosemicarbazones Scientific paper
Main Article Content
Abstract
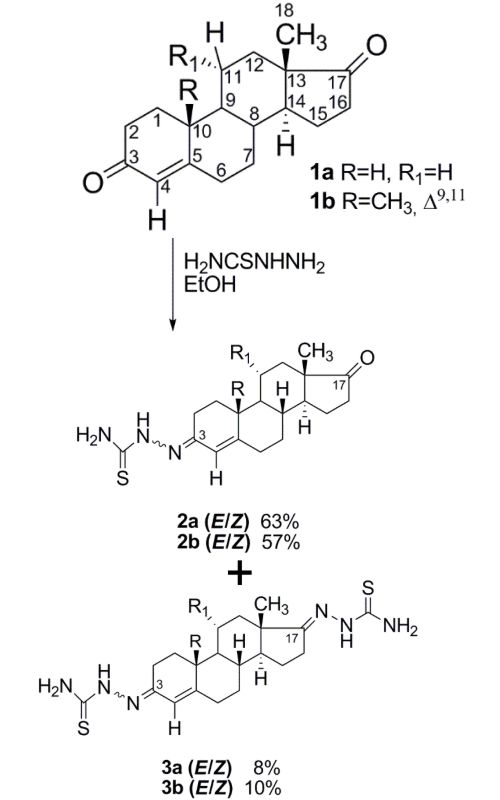
In this work, Pt(II) complexes of previously synthesized steroidal thiosemicarbazones were synthesized and characterized. The ligands and their metal complexes were studied by analytical and spectroscopic data (elemental analysis, IR, 1D-NMR and 2D-NMR, HSQC, HMBC, NOESY, COSY), the analysis of which enabled complete 1H and 13C assignments of each compound including E and Z isomers. All the synthesized ligands and complexes were screened for their cytotoxic and antimicrobial activity. The results demonstrate that the new steroidal thiosemicarbazone complexes were significantly less cytotoxic than the corresponding steroidal thiosemicarbazones. In addition, complexes showed lower antimicrobial activity than the standard drugs, similar to the activity of the starting thiosemicarbazones.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
M. B. Živković, I. T. Novaković, I. Z. Matić, D. M. Sladić, N. M. Krstić, Steroids 148 (2019) 36 (https://doi.org/10.1016/j.steroids.2019.04.010)
S. A. Khan, P. Kumar, R. Joshi, P. F. Iqbal, K. Saleem, Eur. J. Med. Chem. 43 (2008) 2029 (https://doi.org/10.1016/j.ejmech.2007.12.004)
N. Ergenç, N. S. Günaya, R. Demirdamar, Eur. J. Med. Chem. 33 (1998) 143 (https://doi.org/10.1016/S0223-5234(98)80039-1)
T. A. Voronina, N. M. Tozhanova, Farmakol. Toksikol. 44 (1981) 155 (https://www.ncbi.nlm.nih.gov › pubmed)
K. Swathi, M. Sarangapani, World J. Pharm. Pharm. Sci. 3 (2014) 2720 (https://www.wjpps.com/wjpps_controller/archive_show/2014/VOLUME%203,%20FEBRUARY,%20ISSUE%202)
V. Mishra, S. N. Pandeya, Acta Pharm. 51 (2001) 183 (http://acta.pharmaceutica.farmaceut.org/18301.html)
N. Ergenç, G. Çapana, N. S. Günaya, S. Özkırımlı, M. Güngör, S. Özbey, E. Kendic, Arch. Pharm. (Weinheim, Ger.) 332 (1999) 343 (https://doi.org/10.1002/(SICI)1521-4184(199910)332:10<343::AID-ARDP343>3.0.CO;2-0)
S. A. Khan, A. M. Asiri, Chin. J. Chem. 30 (2012) 1901 (https://doi.org/10.1002/cjoc.201200126)
E. Bermejo, R. Carballo, A. Castiñeiras, R. Domínguez, A. E. Liberta, C. Maichle-Mössmer, D. X. West, Z. Naturforsch. 54b (1999) 777 (https://doi.org/10.1515/znb-1999-0613)
S. Singh, F. Athar, M. R. Maurya, A. Azam, Eur. J. Med. Chem. 41 (2006) 592 (https://doi.org/10.1016/j.ejmech.2006.01.014)
M. Asif, A. Husain, J. Appl. Chem. (London, U. K.) 2013 (2013), Article ID 247203 (http://dx.doi.org/10.1155/2013/247203)
K. Verma, S. N. Pandeya, U. K. Singh, S. Gupta, P. Prashant, Anurag, G. Bhardwaj, Int. J. Pharm. Sci. Nanotech. 1 (2008) 357 (https://doi.org/10.37285/ijpsn.2008.1.4.7)
J. A. Lessa, I. C. Mendes, P. R. O. da Silva, M. A. Soares, R. G. dos Santos, N. L. Speziali, N. C. Romeiro, E. J. Barreiro, H. Beraldo, Eur. J. Med. Chem. 45 (2010) 5671 (https://doi.org/10.1016/j.ejmech.2010.09.021)
J. M. Pérez, A. I. Matesanz, A. Martín-Ambite, P. Navarro, C. Alonso, P. Souza, J. Inorg. Biochem. 75 (1999) 255 (https://doi.org/10.1016/S0162-0134(99)00096-3)
L. H. Hall, S. Y. Cheen, J. B. Barnes, D. X. West, Met.-Based Drugs 6 (1999) 143 (http://dx.doi.org/10.1155/MBD.1999.143)
K. H. Reddy, P. S. Reddy, P. R. Babu, J. Inorg. Biochem. 77 (1999) 169 (https://doi.org/10.1016/S0162-0134(99)00188-9)
P. F. Kelly, A. M. Z. Slawin, A. Soriano-Rama, J. Chem. Soc. Dalton Trans. (1972–
–1999) (1996) 53 (https://doi.org/10.1039/DT9960000053)
D. X. West, S. B. Pardhye, P. B. Sonawane, Struct. Bonding (Berlin, Ger.) 76 (1991) 1 (https://doi.org/10.1007/3-540-53499-7_1)
A. E. Liberta, D. X. West, BioМetals 5 (1992) 121 (https://doi.org/10.1007/BF01062223)
D. X. West, A. E. Liberta, S. B. Padhye, R. C. Chikate, P. B. Sonawane, A. S. Kumbhar, R. G. Yerande, Coord. Chem. Rev. 123 (1993) 49 (https://doi.org/10.1016/0010-8545(93)85052-6)
M. B. Živković, I. Z. Matić, M. V. Rodić, I. T. Novaković, D. M. Sladić, N. M. Krstić, RSC Adv. 6 (2016) 34312 (https://doi.org/10.1039/c6ra01516f)
B. Rosenberg, L. Van Camp, T. Krigas, Nature 205 (1965) 698 (https://doi.org/10.1038/205698a0)
B. Rosenberg, L. Van Camp, J. E. Trosko, V. H. Mansour, Nature 222 (1969) 385 (https://doi.org/10.1038/222385a0)
P. J. Loehrer, L. H. Einhorn, Ann. Intern. Med. 100 (1984) 704 (https://doi.org/10.7326/0003-4819-100-5-704)
Z. Guo, P. J. Sadler, Angew. Chem. Int. Ed. 38 (1999) 1512 (https://doi.org/10.1002/(SICI)1521-3773(19990601)38:11<1512::AID-ANIE1512>3.0.CO;2-Y)
A. R. Savić, PhD Thesis, University of Belgrade, Faculty of Chemistry, 2014 (in Serbian) (https://cherry.chem.bg.ac.rs/handle/123456789/2703)
N. M. Krstić, M. S. Bjelaković, M. M. Dabović, V. D. Pavlović, Molecules 15 (2010) 3462 (https://doi:10.3390/molecules15053462)
N. M. Krstić, M. S. Bjelaković, V. D. Pavlović, K. Robeyns, Z. D. Juranić, I. Matić, I. Novaković, D. M. Sladić, Steroids 77 (2012) 558 (https://doi.org/10.1016/j.steroids.2012.02.001)
N. M. Krstić, V. D. Pavlović, I. T. Novaković, I. Z. Matić, D. M. Sladić, Mol. Diversity 17 (2013) 547 (https://doi.org/10.1007/s11030-013-9455-9)
N. M. Krstić, I. Z. Matić, Z. D. Juranić, I. T. Novaković, D. M. Sladić, J. Steroid. Biochem. Mol. Biol. 143 (2014) 365 (http://dx.doi.org/10.1016/j.jsbmb.2014.06.005)
A. Murugkar, B. Unnikrishnan, S. Padhye, R. Bhonde, S. Teat, E.Triantafillou, E. Sinn, Met.-Based Drugs 6 (1999) 177 (http://dx.doi.org/10.1155/MBD.1999.177)
Y. Huang, E. Kong, C. Gan, Z. Liu, Q. Lin, J. Cui, Bioinorg. Chem. Appl. (2015), Article ID 742592 (http://dx.doi.org/10.1155/2015/742592)
T. Mosmann, J. Immunol. Methods 65 (1983) 55 (https://doi:org/10.1016/0022-1759(83)90303-4)
M. Ohno, T. Abe, J. Immunol. Methods 145 (1991) 199 (https://doi:org/10.1016/0022-1759(91)90327-C)
C. Perez, M. Pauli, P. Bazerque, Acta Biol. Med. Exp. 15 (1990) 113
B. N. Meyer, N. R. Ferrigni, J. E. Putnam, L. B. Jacobsen, D. E. Nichols, J. L. McLaughlin, Planta Med. 45 (1982) 31 (http://dx.doi.org/10.1055/s-2007-971236).