UV light impact on phthalates migration from children’s toys into artificial saliva Scientific paper
Main Article Content
Abstract
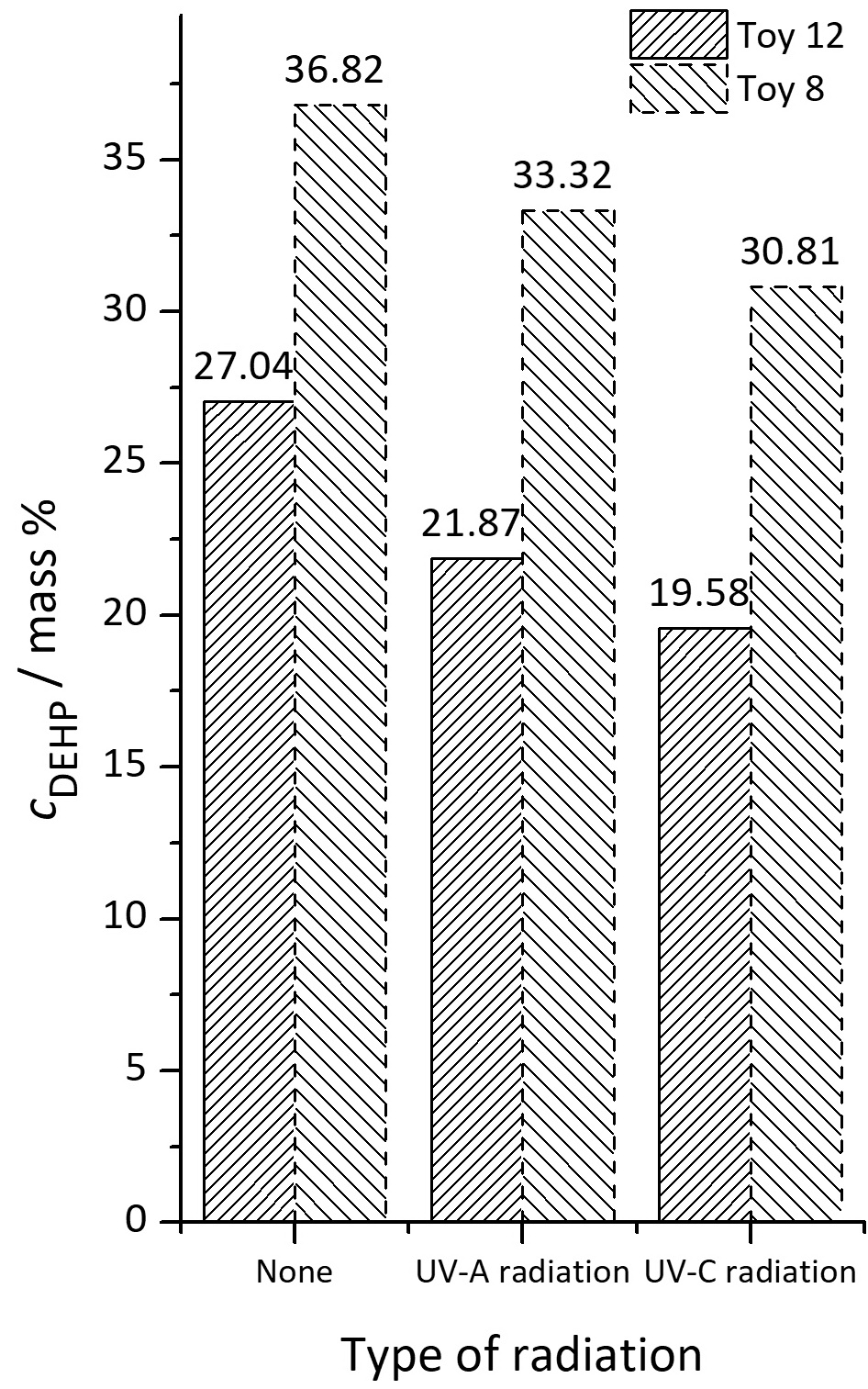
Phthalates has been widely used in children’s toys as plastic plasticizers and softeners. Therefore, attention should be paid to plastic toys, especially those that children can put in their mouths. In this paper quantification of five phthalates: DMP, DnBP, BBP, DEHP and DnOP in plastic toys, as well as irradiation of toys with UV light was performed. After sample preparation and development of the liquid–liquid phthalate extraction method from artificial saliva phthalate quantitative determination using the GC–MS technique was performed. The mean recovery value for DEHP is 77.03±2.76 %. The determination of phthalate in the recipient models (artificial saliva and n-hexane) was performed after 6, 15 and 30 days of the migration test using the GC–MS technique. Based on the known mass % DEHP in the analyzed toys, the percentage of phthalate migration from each analyzed toy to the recipient model after 6, 15 and 30 days of the migration test was calculated. The results show that there is no significant migration of DEHP into artificial saliva, due to high polarity of the recipient (artificial saliva is polar), unlike n-hexane where the migration of DEHP is significant because it is a non-polar solvent.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
S. Net, R. Sempere, A. Delmont, A. Paluselli, B. Ouddane, Environ. Sci. Technol. 49 (2015) 4019 (https://doi.org/10.1021/es505233b)
K. Stamatelatou, C. Pakou, G. Lyberatos, Comprehen. Biotechnol. 6 (2011) 473 (https://doi.org/10.1016/B978-0-08-088504-9.00496-7)
R. Rudel, L. Perovich, Atmos. Environ. 43 (2008) 170 (https://doi.org/10.1016/j.atmosenv.2008.09.025)
M. Criado, V. Fernandez Pinto, A. Badessari, D. Cabral, Int. J. Food Microbiol. 99 (2005) 343 (https://doi.org/10.1016/j.ijfoodmicro.2004.10.036)
I. Kostić, T. Andjelković, D. Andjelković, T.Cvetkovic, D.Pavlović, J. Serb. Chem. Soc. 83(10) (2018) 1157 (https://doi.org/10.2298/JSC180423058K)
E. Gray, J. Ostby, J. Furr, M. Price, R. Veeramachaneni, L. Parks, Toxicol. Sci. 58 (2000) 350 (https://doi.org/10.1093/toxsci/58.2.350)
R. H. Waring, R. M. Harris, Maturitas 68 (2011) 111 (https://doi.org/10.1016/j.maturitas.2010.10.008)
S. S. S. Rowdhwal, J. Chen, BioMed Res. Int. (2018) 1 (https://doi.org/10.1155/2018/1750368)
Directive 2005/84/EC of the Europian Parliament and of the Council, Off. J. Eur. Union, 2005
M. Al-Natsheh, M. Alawi, M. Fayyada, I. Tarawneh, J. Chrom., B 985 (2015) 103 (https://doi.org/10.1016/j.jchromb.2015.01.010)
T. Yuzawa, C. Watanabe, R. Freeman, S. Tsuge, Anal. Sci. 25 (2009) 1057 (https://doi.org/10.2116/analsci.25.1057)
M. Akkbik, V. Ali Turksoy, S. Koçoğlu, Toxicol. Mech. Methods 30 (2020) 33 (https://doi.org/10.1080/15376516.2019.1650145)
U. Hauri, U. Schlegel, M. Wagmann, C. Hohl, Mitt. Gebiete Lebensm. Hyg. 93 (2002) 179
Standard operation procedure for Determination of release of phthalate plasticizers in saliva simulant, Appendix 1 of report V3932, Ref. Ares 4242543, 2015
R. Hampton, J. Havel, Introductory biological statistics, Waveland Press, Long Grove, IL, 2006, pp. 99–120.