New rhodium(III)–ED3AP complex: Crystal structure, characterization and computational chemistry Scientific paper
Main Article Content
Abstract
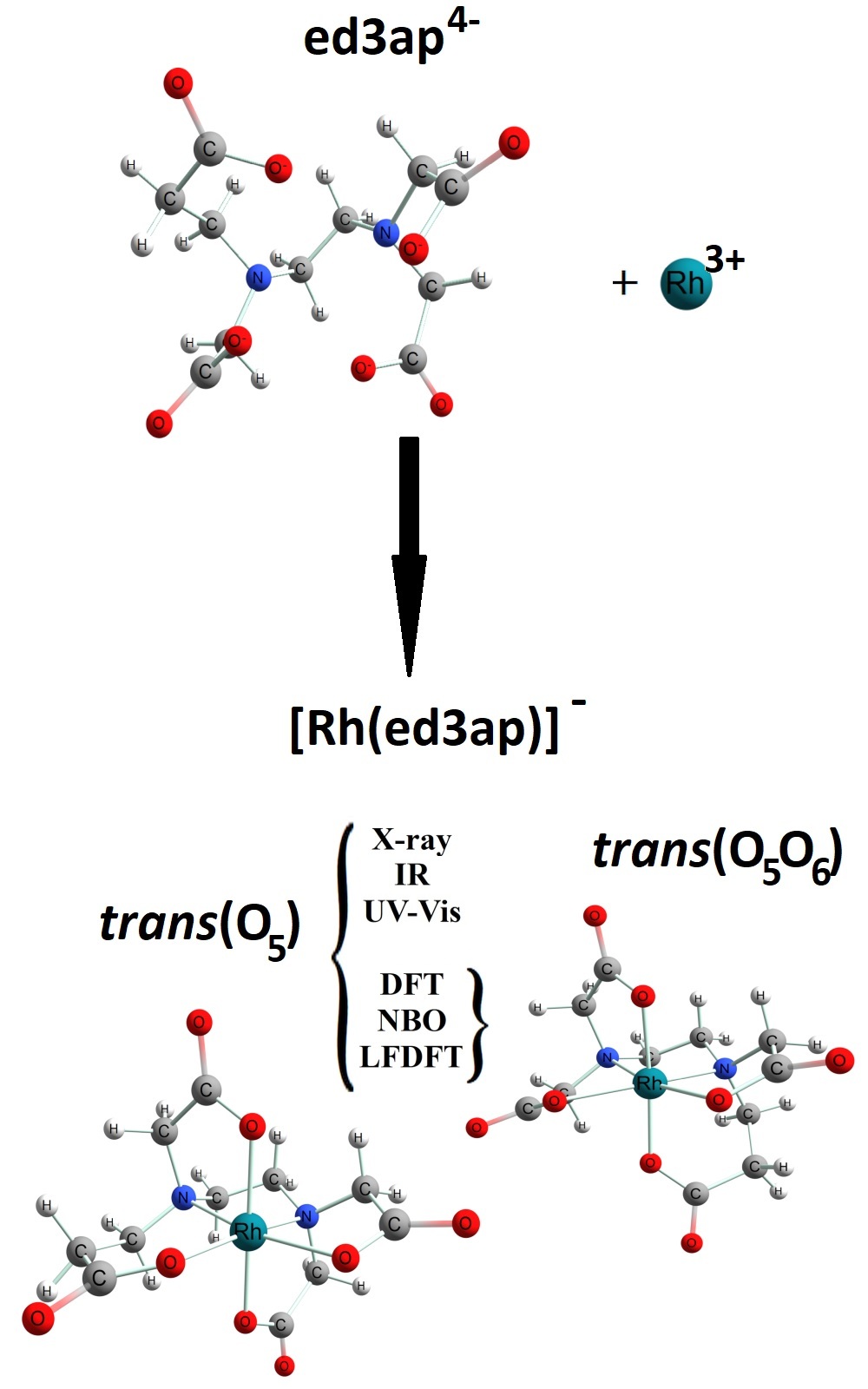
Only one (trans(O5)-Na[Rh(ED3AP)]∙3H2O) of possible two isomers was synthesized and characterized by single crystal X-ray analysis, IR and UV–Vis spectroscopy. Computational analysis of both isomers was performed with three levels of theory (B3LYP/TZV, BP86/TZV, OPBE/TZV), which gave consistent results. The more stable isomer by total energy and ligand field stabilization energy (LFSE) was trans(O5) which appeared in synthesis. The calculation of excited state energies complied with UV–Vis spectra, especially with OPBE functional. The results of excited state energy pointed out the differences among isomers in means of a splitting pattern of 1T2g excited state term. Both isomers have a strongly delocalized structure, according to the natural bonding orbital (NBO) analysis. NBO analysis shows that the trans(O5) isomer is more stable than trans(O5O6) for approx. 87 kJ/mol. Therefore, only the trans(O5) isomer is present in the reaction mixture.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-9/2021-14/200122; 451-03-9/2021-14/200026
References
C. Drouza, M. Vlasiou, A. D. Keramidas, Dalt. Trans. 42 (2013) 11831 (https://doi.org/10.1039/C3DT50619C)
E. Repo, J. K. Warchoł, A. Bhatnagar, M. Sillanpää, J. Colloid Interface Sci. 358 (2011) 261 (https://doi.org/10.1016/j.jcis.2011.02.059)
M. E. Markowitz, J. F. Rosen, J. Pediatr. 119 (1991) 305 (https://doi.org/10.1289/ehp.99107437)
K. Sakthithasan, P. Lévy, J. Poupon, R. Garnier, Clin. Toxicol. 56 (2018) 1143 (https://doi.org/10.1080/15563650.2018.1478424)
G. A. Lamas, O. M. Issa, Curr. Cardiol. Rep. 18 (2016) 20 (https://doi.org/10.1007/s11886-015-0690-9)
F. G. Kari, W. Giger, Environ. Sci. Technol. 29 (1995) 2814 (https://doi.org/10.1021/es00011a018)
H. Xue, L. Sigg, F. Günter Kari, Environ. Sci. Technol. 29 (1995) 59 (https://doi.org/10.1021/es00001a007)
J. Porath, J. Carlsson, I. Olsson, G. Belfrage, Nature 258 (1975) 598 (https://doi.org/10.1038/258598a0)
J. Carrasco-Castilla, A. J. Hernández-Álvarez, C. Jiménez-Martínez, C. Jacinto-Hern-ández, M. Alaiz, J. Girón-Calle, J. Vioque, G. Dávila-Ortiz, Food Chem. 135 (2012) 1789 (https://doi.org/10.1016/j.foodchem.2012.06.016)
M. S. Jeremić, M. D. Radovanović, F. W. Heinemann, M. M. Vasojević, Z. D. Matović, Polyhedron 169 (2019) 89 ( https://doi.org/10.1016/j.poly.2019.04.053)
M. S. Jeremić, H. Wadepohl, V. V. Kojić, D. S. Jakimov, R. Jelić, S. Popović, Z. D. Matović, P. Comba, RSC Adv. 7 (2017) 5282 (https://doi.org/10.1039/C6RA26199J)
M. S. Jeremić, M. D. Radovanović, F. Bisceglie, V. V. Kojić, R. Jelić, Z. D. Matović, Polyhedron 156 (2018) 19 (https://doi.org/10.1016/j.poly.2018.08.075)
K. D. Gailey, D. J. Radanović, M. Djuran, B. E. Douglas, J. Coord. Chem. 8 (1978) 161 (https://doi.org/10.1080/00958977808073090)
D. J. Radanovic, K. D. Gailey, M. I. Djuran, B. E. Douglas, J. Coord. Chem. 10 (1980) 115 (https://doi.org/10.1080/00958978008079858)
D. J. Radanovic, V. D. Miletic, T. Ama, H. Kawaguchi, Bull. Chem. Soc. Jpn. 71 (1998) 1605 (https://doi.org/10.1246/bcsj.71.1605)
D. J. Radanović, N. Sakagami, V. M. Ristanović, S. Kaizaki, Inorganica Chim. Acta 292 (1999) 16 (https://doi.org/10.1016/S0020-1693(99)00164-4)
D. J. Radanovic, T. Ama, D. M. Gurešic, D. M. Ristanovic, D. D. Radanovic, H. Kawaguchi, Bull. Chem. Soc. Jpn. 73 (2000) 2283 (https://doi.org/10.1246/bcsj.73.2283)
V. D. Miletić, A. Meetsma, P. J. van Koningsbruggen, Z. D. Matović, Inorg. Chem. Commun. 12 (2009) 720 (https://doi.org/10.1016/j.inoche.2009.05.029)
R. Pettinari, F. Marchetti, C. Pettinari, F. Condello, A. Petrini, R. Scopelliti, T. Riedel, P. J. Dyson, Dalt. Trans. 44 (2015) 20523 (https://doi.org/10.1039/C5DT03037D)
F. Hackenberg, L. Oehninger, H. Alborzinia, S. Can, I. Kitanovic, Y. Geldmacher, M. Kokoschka, S. Wölfl, I. Ott, W. S. Sheldrick, J. Inorg. Biochem. 105 (2011) 991 (https://doi.org/10.1016/j.jinorgbio.2011.04.006)
S. Mukhopadhyay, R. K. Gupta, R. P. Paitandi, N. K. Rana, G. Sharma, B. Koch, L. K. Rana, M. S. Hundal, D. S. Pandey, Organometallics 34 (2015) 4491 (https://doi.org/10.1021/acs.organomet.5b00475)
S. Mollin, R. Riedel, K. Harms, E. Meggers, J. Inorg. Biochem. 148 (2015) 11 (https://doi.org/10.1016/j.jinorgbio.2015.01.005)
T.-S. Kang, W. Wang, H.-J. Zhong, J.-X. Liang, C.-N. Ko, J.-J. Lu, X.-P. Chen, D.-L. Ma, C.-H. Leung, Biochim. Biophys. Acta - Gen. Subj. 1861 (2017) 256 (https://doi.org/10.1016/j.bbagen.2016.11.032)
A. Lapasam, V. Banothu, U. Addepally, M. R. Kollipara, J. Mol. Struct. 1191 (2019) 314 (https://doi.org/10.1016/j.molstruc.2019.04.116)
A. Lapasam, L. Dkhar, N. Joshi, K. M. Poluri, M. R. Kollipara, Inorg. Chim. Acta 484 (2019) 255 (https://doi.org/10.1016/j.ica.2018.09.067)
L. Shadap, S. Diamai, V. Banothu, D. P. S. Negi, U. Adepally, W. Kaminsky, M. R. Kollipara, J. Organomet. Chem. 884 (2019) 44 (https://doi.org/10.1016/j.jorganchem.2019.01.019)
S. S. Hassan, Appl. Organomet. Chem. 32 (2018) e4170 (https://doi.org/10.1002/aoc.4170)
E. D. Glendening, C. R. Landis, F. Weinhold, J. Comput. Chem. 34 (2013) 2134 (https://doi.org/10.1002/jcc.23366)
M. Atanasov, C. A. Daul, C. Rauzy, Chem. Phys. Lett. 367 (2003) 737 (https://doi.org/10.1016/S0009-2614(02)01762-1)
SADABS v. 2.06, Bruker AXS, Inc., Madison, WI, 2002
G. M. Sheldrick, Acta Cryst. A 64 (2008) 112 (https://doi.org/10.1107/S0108767307043930)
G.M. Sheldrick, Acta Cryst. C 71 (2015) 3 (https://doi.org/10.1107/S2053229614024218)
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, H. Puschmann, J. Appl. Crystallogr. 42 (2009) 339 (https://doi.org/10.1107/S0021889808042726)
Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016 (https://gaussian.com/relnotes)
P. J. Stephens, F. J. Devlin, C. F. Chabalowski, M. J. Frisch, J. Phys. Chem. 98 (1994) 11623 (https://doi.org/10.1021/j100096a001)
M. F. Peintinger, D. V. Oliveira, T. Bredow, J. Comput. Chem. 34 (2013) 451 (https://doi.org/10.1002/jcc.23153)
G. te Velde, F. M. Bickelhaupt, E. J. Baerends, C. Fonseca Guerra, S. J. A. van Gisbergen, J. G. Snijders, T. Ziegler, J. Comput. Chem. 22 (2001) 931 (https://doi.org/10.1002/jcc.1056)
J. P. Perdew, Phys. Rev. B 34 (1986) 7406 (https://doi.org/10.1103/PhysRevB.34.7406)
J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 77 (1996) 3865 (https://doi.org/10.1103/PhysRevLett.77.3865)
D. Cremer, J. A. Pople, J. Am. Chem. Soc. 97 (1975) 1354 (https://doi.org/10.1021/ja00839a011).