Synthesis and structural characterization of Cd(II) complexes with 2-acetylpyridine-aminoguanidine – a novel coordination mode Scientific paper
Main Article Content
Abstract
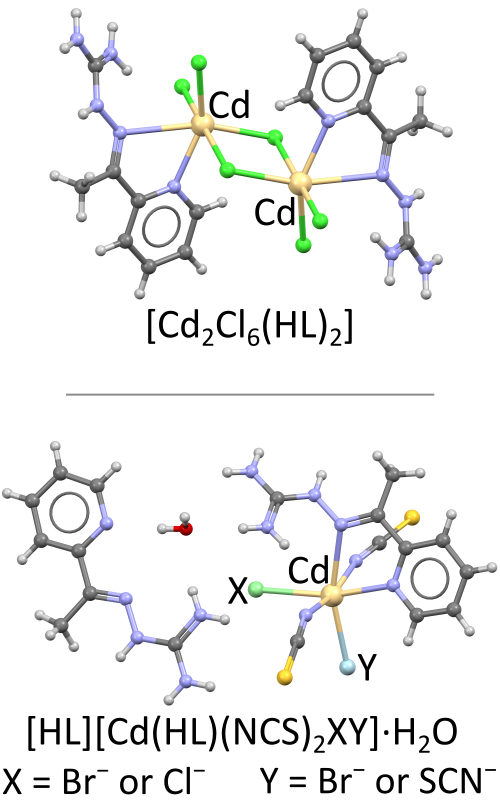
The structures of the first two complexes with bidentate coordination of aminoguanidine Schiff base, i.e., 2-acetylpyridine-aminoguanidine (L), are reported. The complex of the formula [Cd2Cl6(HL)2] (1) was obtained in the reaction of warm aqueous solutions of chloride salts of Cd(II), Zn(II), and the ligand, while the reaction of cadmium bromide and the ligand in the presence of deprotonating agent as well as ammonium thiocyanate resulted in the formation of the complex in which Schiff base has both the role of the ligand and the counterion, viz. [HL][Cd(HL)(NCS)2XY]·H2O (2), where X = Cl−/Br−, and Y = Br−/SCN–. The complexes were characterized by IR spectroscopy, elemental analysis, conductometric measurements, and SC-XRD. The unusual bidentate coordination of the Schiff base lead to significant changes in the geometry of this molecule (from almost planar in free form and as a tridentate ligand to twisted as a bidentate ligand). Besides, in complex 1 relatively rare bridging coordination of Cl– anions in octahedral Cd(II) is found, while the crystal structure of complex 2 exhibits substitutional disorder, and contains four different anions: [Cd(HL)(NCS)2Br(SCN)]− (ca. 61 %), [Cd(HL)(NCS)2Cl(SCN)]− (ca. 35 %), [Cd(HL)(NCS)2Br2]− (ca. 3 %), and [Cd(HL)(NCS)2ClBr] − (ca. 1 %).
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-9/2021-14/200125
References
R. Hernández-Molina, A. Mederos, in Comprehensive Coordination Chemistry II, J. A. McCleverty, T. J. Meyerpp, Eds., Elsevier, Amsterdam, 2003, pp. 411–446 (https://doi.org/10.1016/B0-08-043748-6/01070-7)
K. C. Gupta, A. K. Sutar, Coord. Chem. Rev. 252 (2008) 1420 (https://doi.org/10.1016/j.ccr.2007.09.005)
J. Zhang, L. Xu, W.-Y. Wong, Coord. Chem. Rev. (2017) (https://doi.org/10.1016/j.ccr.2017.08.007)
S. Saeednia, P. Iranmanesh, M. H. Ardakani, M. Mohammadi, Gh. Norouzi, Mat. Res. Bull. 78 (2016) 1 (https://doi.org/10.1016/j.materresbull.2016.02.010)
H.-B. Yang, K. Ghosh, Y. Zhao, B. H. Northrop, M. M. Lyndon, D. C. Muddiman, H. S. White, P. J. Stang, J. Am. Chem. Soc. 130 (2008) 839 (https://doi.org/10.1021/JA710349J)
P. A. Vigato, S. Tamburini, L. Bertolo, Coord. Chem. Rev. 251 (2007) 1311 (https://doi.org/10.1016/J.CCR.2006.11.016)
S. Satapathi, S. Das, K. Bhar, R. K. Kumar, T. K. Maji, B. K. Ghosh, Polyhedron 30 (2011) 387 (https://doi.org/10.1016/j.poly.2010.11.006)
S. Das, K. Bhar, S. Chattopadhyay, P. Mitra, V. J. Smith, L. J. Barbour, B. K. Ghosh, Polyhedron 38 (2012) 26 (https://doi.org/10.1016/J.POLY.2012.02.013)
S. Manna, E. Zangrando, S. C. Manna, Polyhedron 177 (2020) 114296 (https://doi.org/10.1016/J.POLY.2019.114296)
C. L. Siewit, B. Gengler, E. Vegas, R. Puckett, M. C. Louie, Mol. Endocrinol. 24 (2010) 981 (https://doi.org/10.1210/ME.2009-0410)
P. Joseph, Toxicol. Appl. Pharm. 238 (2009) 272 (https://doi.org/10.1016/J.TAAP.2009.01.011)
M. Filipič, Mut. Res. 733 (2012) 69 (https://doi.org/10.1016/J.MRFMMM.2011.09.002)
K. Golovine, P. Makhov, R. G. Uzzo, A. Kutikov, D. J Kaplan, E. Fox, V. M. Kolenko, Mol. Cancer 9 (2010) (https://doi.org/10.1186/1476-4598-9-183)
C. Casano, M. Agnello, R. Sirchia, C. Luparello, Biometals 23 (2010) 83 (https://doi.org/10.1007/S10534-009-9268-6)
N. Zhang, Y. Fan, Z. Zhang, J. Zuo, P. Zhang, Q. Wang, S. Liu, C. Bi, Inorg. Chem. Commun. (2012) 68 (https://doi.org/10.1016/J.INOCHE.2012.05.022)
N. Zhang, Y. Fan, G. Huang, D. Buac, C. Bi, Y. Ma, X. Wang, Z. Zhang, X. Zhang, Q. P. Dou, Inorg. Chim. Acta 466 (2017) 478 (https://doi.org/10.1016/J.ICA.2017.07.006)
Lj. S. Vojinović-Ješić, M. M. Radanović, M. V. Rodić, V. Živković-Radovanović, L. S. Jovanović, V. M. Leovac, Polyhedron 117 (2016) 526 (https://doi.org/10.1016/j.poly.2016.06.032)
Bruker APEX2, SAINT, and SADABS.Bruker AXS Inc., Madison, WI, 2009 (n.d.)
Rigaku Oxford Diffraction, CrysAlisPro Software system, Rigaku Corporation, Oxford, 2018
G. M. Sheldrick, Acta Crystallogr., A 71 (2015) 3 (https://doi.org/10.1107/S2053273314026370)
G. M. Sheldrick, Acta Crystallogr., C 71 (2015) 3 (https://doi.org/10.1107/S2053229614024218)
M. M. Radanović, M. V. Rodić, Lj. S. Vojinović-Ješić, S. Armaković, S. J. Armaković, V. M. Leovac, Inorg. Chim. Acta 473 (2018) 160 (https://doi.org/10.1016/J.ICA.2017.12.038)
J.-Y. Miao, Acta Crystallogr., E 66 (2010) m868 (https://doi.org/10.1107/S1600536810025225)
C. R. Groom, I. J. Bruno, M. P. Lightfoot, S. C. Ward, Acta Crystallogr., B 72 (2016) 171 (https://doi.org/10.1107/S2052520616003954)
O. Yu. Vassilyeva, E. A. Buvaylo, V. N. Kokozay, S. L. Studzinsky, B. W. Skelton, G. S. Vasyliev, Acta Crystallogr., E 78 (2022) 173 (https://doi.org/10.1107/S2056989022000317)
G. R. Andrade, J. Kunsminskas, L. Pizzuti, A. dos Anjos, S. D. Inglez, B. Tirloni, P. H. Suegama, Inorg. Chem. Commun. 61 (2015) 210 (https://doi.org/10.1016/j.inoche.2015.09.022)
Z. A. Starikova, A. I. Yanovsky, Yu. T. Struchkov, S. V. Zubkov, I. I. Seifullina, Russ. Chem. Bull. (1996) 2157 (https://doi.org/10.1007/BF01430730)
B. W. Skelton, V. N. Kokozay, O. Yu. Vassilyeva, E. A. Buvaylo, CSD Commun., Database Identifier GOTFIH, Deposition Number 1959073 (2019) (https://doi.org/10.5517/ccdc.csd.cc23rkxc)
O. T. Ujam, S. M. Devoy, W. Henderson, B. K. Nicholson, T. S. A. Hor, Inorg. Chim. Acta 363 (2010) 3558 (https://doi.org/10.1016/j.ica.2010.07.011)
M. M. Radanović, S. B. Novaković, Lj. S. Vojinović-Ješić, M. V. Rodić, V. M. Leovac, J. Serb. Chem. Soc. 83 (2018) 157 (https://doi.org/10.2298/JSC170922116R)
Q. Li, H.-T. Wang, L. Zhou, Acta Crystallogr., C 71 (2015) 93 (https://doi.org/10.1107/S2053229614028009)
S. Khanna, S. Verma, CrystEngComm 16 (2014) 6680 (https://doi.org/10.1039/C4CE00611A)
Á. Garcı́a-Raso, J. J. Fiol, F. Bádenas, X. Solans, M. Font-Bardia, Polyhedron 18 (1999) 765 (https://doi.org/10.1016/S0277-5387(98)90351-5)
P. R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka, M. A. Spackman, J. Appl. Crystallogr. 54 (2021) 1006 (https://dx.doi.org/10.1107/S1600576721002910).