Impedance response of aluminum alloys with varying Mg content in Al–Mg systems during exposure to chloride corrosion environment Scientific paper
Main Article Content
Abstract
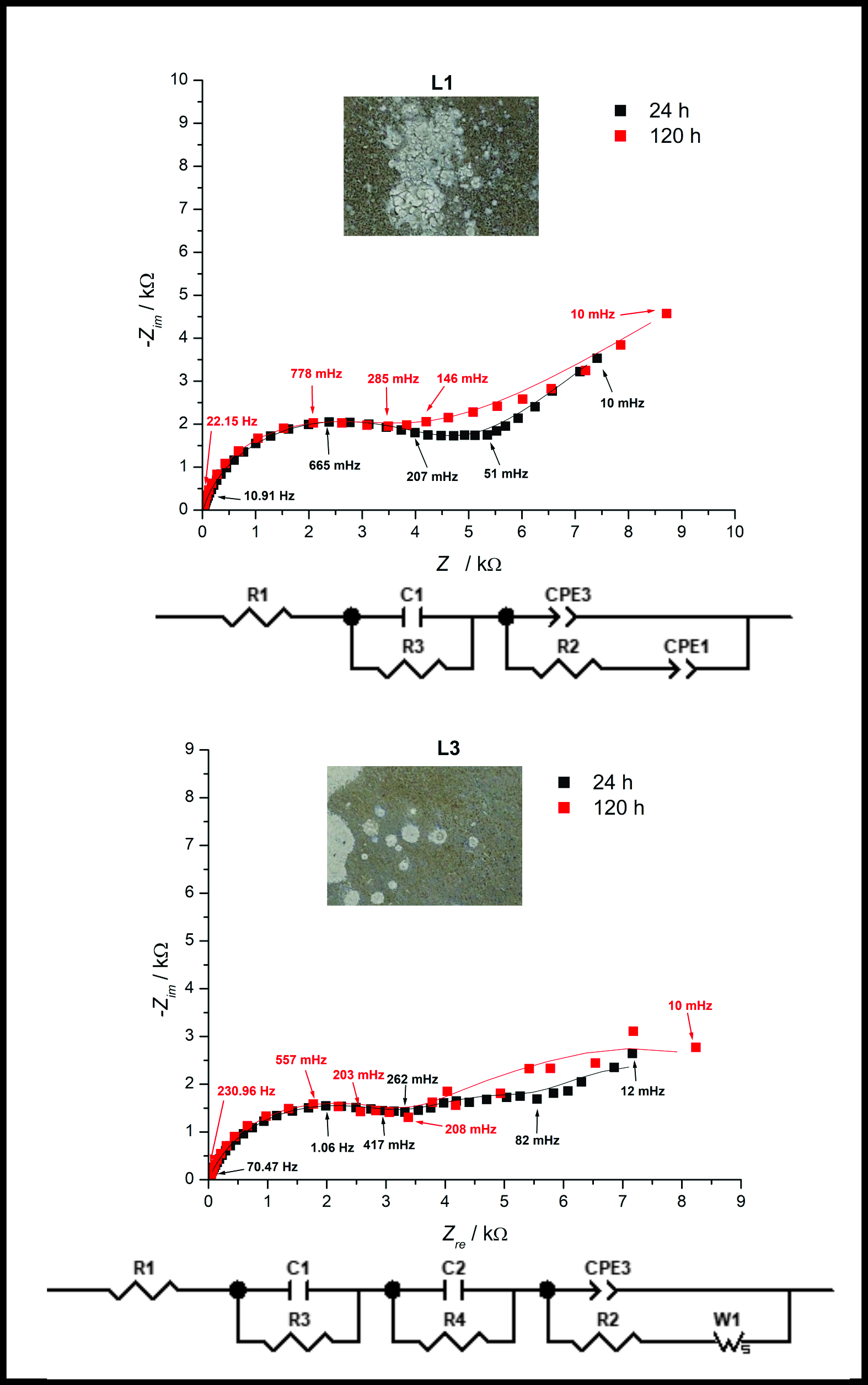
This research discusses the corrosion behavior of as-cast Al alloys with different Mg content by potentiostatic electrochemical impedance spectroscopy (PEIS). The complex plane spectra of all samples feature a high-frequency loop, followed by semi-infinite diffusion impedance characteristics at low frequencies, with the corrosion-induced formation of a defined porous structure of a layer making finite diffusion through the pores dominant upon prolonged exposure. The most compact layer causes the most pronounced and well-resolved finite diffusion features in the impedance spectra of the sample with the highest Mg content, while the sample with the lowest Mg content has a highly porous layer unable to slow down the corrosion rate at the layer/sample interface. The highest layer capacitance and diffusion admittance are found in the sample with the highest Mg content, with a more adherent protective film expected to form. However, the growth rate of the layer was not adequate for the remarkable closing of the pits, indicating the weakness of this sample towards pit activity. The results show that increasing Mg content improves corrosion resistance and clearly separates bulky corrosion from localized pitting corrosion, but it also increases the thickness of a more compact, poorly adhesive layer.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200026
References
X. Zhang, M. Zhang, R. Li, X. Feng, X. Pang, J. Rao, D. Cong, C. Yin, Y. Zhang, Coatings 11 (2021) (https://doi.org/10.3390/coatings11111316)
K. A. Yasakau, M. L. Zheludkevich, S. V Lamaka, M. G. S. Ferreira, Electrochim. Acta 52 (2007) 7651 (https://doi.org/10.1016/j.electacta.2006.12.072)
L. Garrigues, N. Pebere, F. Dabosi, Electrochim. Acta 41 (1996) 1209 (https://doi.org/10.1016/0013-4686(95)00472-6)
S. O. Adeosun, O. I. Sekunowo, S. A. Balogun, V. D. Obiekea, Int. J. Corros. 2012 (2012) 927380 (https://doi.org/10.1155/2012/927380)
S. P. B., T. U. Dhanaji, S. Dassani, M. Somasundaram, A. Muthuchamy, A. Raja Annamalai, Crystals 13 (2023) (https://doi.org/10.3390/cryst13020344)
B. Li, Z. Zhang, T. Liu, Z. Qiu, Y. Su, J. Zhang, C. Lin, L. Wang, Materials (Basel, Switzerland) 15 (2022) (https://doi.org/10.3390/ma15113912)
S. Ren, X. He, X. Qu, I. S. Humail, Y. Li, Mater. Sci. Eng., B 138 (2007) 263 (https://doi.org/10.1016/j.mseb.2007.01.023)
F. Andreatta, H. Terryn, J. H. W. de Wit, Corros. Sci. 45 (2003) 1733 (https://doi.org/10.1016/S0010-938X(03)00004-0)
M. Popović, E. Romhanji, Mater. Sci. Eng., A 492 (2008) 460 (https://doi.org/10.1016/j.msea.2008.04.001)
L. Ren, H. Gu, W. Wang, S. Wang, C. Li, Z. Wang, Y. Zhai, P. Ma, Materials (Basel, Switzerland) 12 (2019) (https://doi.org/10.3390/ma12244160)
H. P. Godard, The corrosion of light metals, Wiley, New York, 1967, ISBN: 978-0471308614
А. D. Cristian, M. M. Georgiana, S. A. Victor, M. M. A. B. Abdullah, AIP Conf. Proc. 1835 (2017) 20051 (https://doi.org/10.1063/1.4983791)
N. Loukil, in Magnesium Alloys Structure and Properties, T. Tański, P. Jarka, Eds., IntechOpen, Rijeka, 2021, p. 833 (https://doi.org/10.5772/intechopen.96232)
S. K. Kairy, P. A. Rometsch, K. Diao, J. F. Nie, C. H. J. Davies, N. Birbilis, Electrochim. Acta 190 (2016) 92 (https://doi.org/10.1016/j.electacta.2015.12.098)
F. Song, X. Zhang, S. Liu, Q. Tan, D. Li, Corros. Sci. 78 (2014) 276 (https://doi.org/10.1016/j.corsci.2013.10.010)
C. Brito, T. Vida, E. Freitas, N. Cheung, J. E. Spinelli, A. Garcia, J. Alloys Compd. 673 (2016) 220 (https://doi.org/10.1016/j.jallcom.2016.02.161)
M. M. Tawfik, M. M. Nemat-Alla, M. M. Dewidar, J. Mater. Res. Technol. 13 (2021) 754 (https://doi.org/https://doi.org/10.1016/j.jmrt.2021.04.076)
J. Liu, M.-J. Tan, A.-E.-W. Jarfors, Y. Aue-u-lan, S. Castagne, Mater. Des. 31 (2010) S66 (https://doi.org/10.1016/j.matdes.2009.10.052)
J. H. W. de Wit, Electrochim. Acta 49 (2004) 2841 (https://doi.org/10.1016/j.electacta.2004.01.045)
N. Birbilis, R. G. Buchheit, J. Electrochem. Soc. 152 (2005) B140 (https://doi.org/10.1149/1.1869984)
J. Wloka, G. Bürklin, S. Virtanen, Electrochim. Acta 53 (2007) 2055 (https://doi.org/10.1016/j.electacta.2007.09.004)
А. Pardo, M. C. Merino, R. Arrabal, S. Merino, F. Viejo, A. E. Coy, Appl. Surf. Sci. 252 (2006) 2794 (https://doi.org/10.1016/j.apsusc.2005.04.023).