Deashing and desulfurization of subbituminous coal from the East field (Bogovina Basin, Serbia) – Insights from chemical leaching Scientific paper
Main Article Content
Abstract
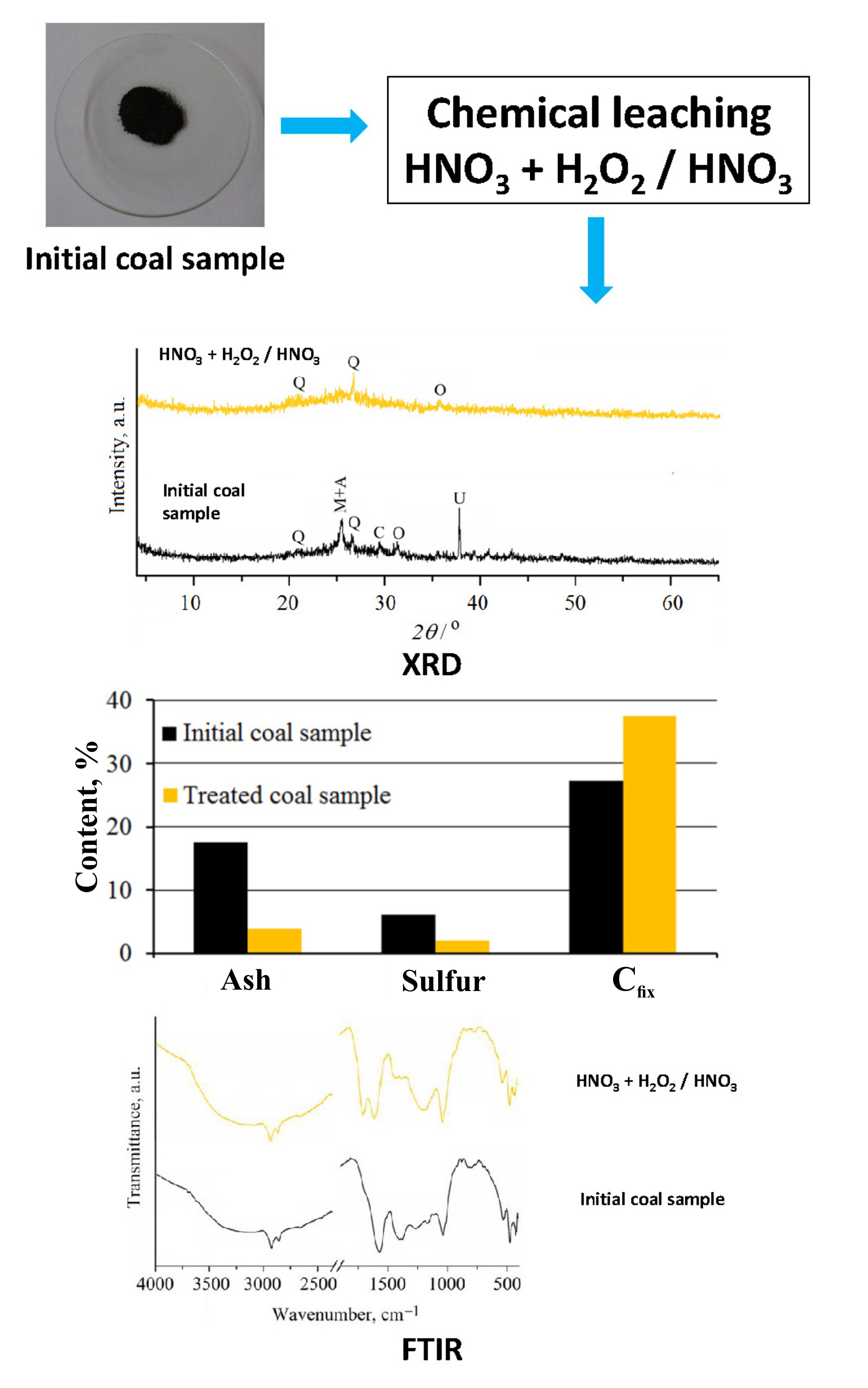
The study is focused on the determination of the most effective chemical leaching process for the simultaneous demineralization/deashing and desulfurization of subbituminous coal from the Bogovina Basin. Coal was treated for 30 min, at different temperatures, using variable concentrations of hydrochloric, nitric, acetic and citric acids; hydrogen peroxide, mixture of hydrogen peroxide and nitric acid (pH 2), as well as by the stepwise leaching process (nitric acid + mixture of hydrogen peroxide and nitric acid, pH 2). The changes in mineral composition, caused by the chemical leaching, are followed using X-ray diffraction, whereas alterations of coal organic matter are tracked by Fourier-transform infrared spectroscopy and the content of fixed carbon. Inorganic acid leaching, regardless of the temperature and acid concentration, enabled the successful deashing of coal, whereas the percent of desulfurization was insufficient. The organic acid leaching was not satisfactory for both, deashing and desulfurization. Leaching by H2O2 and H2O2/HNO3 mixture (pH 2) resulted in moderate desulfurization, but the ash reduction was low. The most suitable method for the simultaneous effective ash (78 wt.%) and the sulfur (66 wt. %) removal from Bogovina coal is the two-step leaching, combining 10 vol. % HNO3 and mixture of 35 vol. % H2O2/10 vol. % HNO3 of pH 2 at 60 °C.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
H. Dhawan, D. K. Sharma, Int. J. Coal Sci. Technol. 6 (2019) 169 (https://doi.org/10.1007/s40789-019-0253-6)
K. Goto, K. Yogo, T. Higashii, Appl. Energy 111 (2013) 710 (https://doi.org/10.1016/j.apenergy.2013.05.020)
F. Rubiera, A. Arenillas, B. Arias, J. J. Pis, I. Suárez-Ruiz, K. M. Steel, J. W. Patrick, Fuel 82 (2003) 2145 (https://doi.org/10.1016/S0016-2361(03)00181-9)
D. Životić, Coal Geology, University of Belgrade, Faculty of Mining and Geology, Bel¬grade, 2018 (in Serbian) (https://rgf.bg.ac.rs/Dokumenta/Publikacije/Biblioteka/Promocija_izdanja_katalog_2019.pdf)
A. Ali, S. K. Srivastava, R. Haque, Fuel 71 (1992) 835 (https://doi.org/10.1016/0016-2361(92)90139-F)
P. Meshram, B. K. Purohit, M. K. Sinha, S. K. Sahu, B. D. Pandey, Renew. Sustain. Energy Rev. 41 (2015) 745 (http://doi.org/10.1016/j.rser.2014.08.072)
D. K. Sharma, G. Wadhwa, World J. Microbiol. Biotechnol. 13 (1997) 29 (https://doi.org/10.1007/BF02770804)
M. Rahman, D. Pudasainee, R. Gupta, Fuel Process. Technol. 158 (2017) 35 (http://doi.org/10.1016/j.fuproc.2016.12.010)
M. Abdollahy, A. Z. Moghaddam, K. Rami, Fuel 85 (2006) 1117 (https://doi.org/10.1016/j.fuel.2005.10.011)
H. G. Alam, A. Z. Moghaddam, M. R. Omidkhah, Fuel Process. Technol. 90 (2009) 1 (http://doi.org/10.1016/j.fuproc.2008.06.009)
J. M. Andrés, A. C. Ferrando, L. Membrado, Energy Fuels 10 (1996) 425 (https://doi.org/10.1021/ef9501612)
K. M. Steel, J. Besida, T. A. ODonnell, D. G. Wood, Fuel Process. Technol. 70 (2001) 193 (https://doi.org/10.1016/S0378-3820(01)00173-4)
K. M. Steel, J. W. Patrick, Fuel 82 (2003) 1917 (https://doi.org/10.1016/S0016-2361(03)00149-2)
E. Jorjani, H. G. Chapi, M. T. Khorami, Fuel Process. Technol. 92 (2011) 1898 (http://doi.org/10.1016/j.fuproc.2011.05.008)
K. M. Steel, J. Besida, T. A. ODonnell, D.G. Wood, Fuel Process. Technol. 70 (2001) 171 (https://doi.org/10.1016/S0378-3820(01)00171-0)
S. Mukherjee, S. K. Srivastava, Energy Fuels 18 (2004) 1764 (https://doi.org/10.1021/ef0499731)
W. Li, E.H. Cho, Energy Fuels 19 (2005) 499 (https://doi.org/10.1021/ef0400767)
H. Karaca, K. Ceylan, Fuel Process. Technol. 50 (1997) 19 (https://doi.org/10.1016/S0378-3820(96)01042-9)
A. A. Yahya, N. Ali, N. L. Mohd Kamal, S. Shahidan, S. Beddu, M. F. Nuruddin, N. Shafiq, MATEC Web Conf. 103 (2017) 01004 (https://doi.org/10.1051/matecconf/201710301004)
J. Wang, A. Tomita, Ind. Eng. Chem. Res. 36 (1997) 1464 (https://doi.org/10.1021/ie960516k)
S. Mukherjee, P. C. Borthakur, Fuel 80 (2001) 2037 (https://doi.org/10.1016/S0016-2361(01)00094-1)
I. C. Cardona, M. A. Márquez, Fuel Process. Technol. 90 (2009) 1099 (https://doi.org/10.1016/j.fuproc.2009.04.022)
Energy Resources of the Republic of Serbia, http://www.smeits.rs/include/data/docs0066.doc (last accessed July 19, 2021) (in Serbian)
D. Životić, B. Jovančićević, J. Schwarzbauer, O. Cvetković, I. Gržetić, M. Ercegovac, K. Stojanović, A. Šajnović, Int. J. Coal Geol. 81 (2010) 227 (https://doi.org/10.1016/j.coal.2009.07.012)
N. Vuković, D. Životić, J. G. Mendonça Filho, T. Kravić-Stevović, M. Hámor-Vidó, J. O. Mendonça, K. Stojanović, Int. J. Coal Geol. 154-155 (2016) 213 (http://doi.org/10.1016/j.coal.2016.01.007)
ASTM D3172: Standard Practice for Proximate Analysis of Coal and Coke , 2013
ISO 334: Solid mineral fuels – Determination of total sulfur – Eschka method, 2013
S. K. Behera, S. Chakraborty, B. C. Meikap, Int. J. Coal Sci. Technol. 5 (2018) 142 (https://doi.org/10.1007/s40789-018-0208-3).