The effect of non-specific binding of Pd(II) complexes with N-heteroaromatic hydrazone ligands on the protein structure Scientific paper
Main Article Content
Abstract
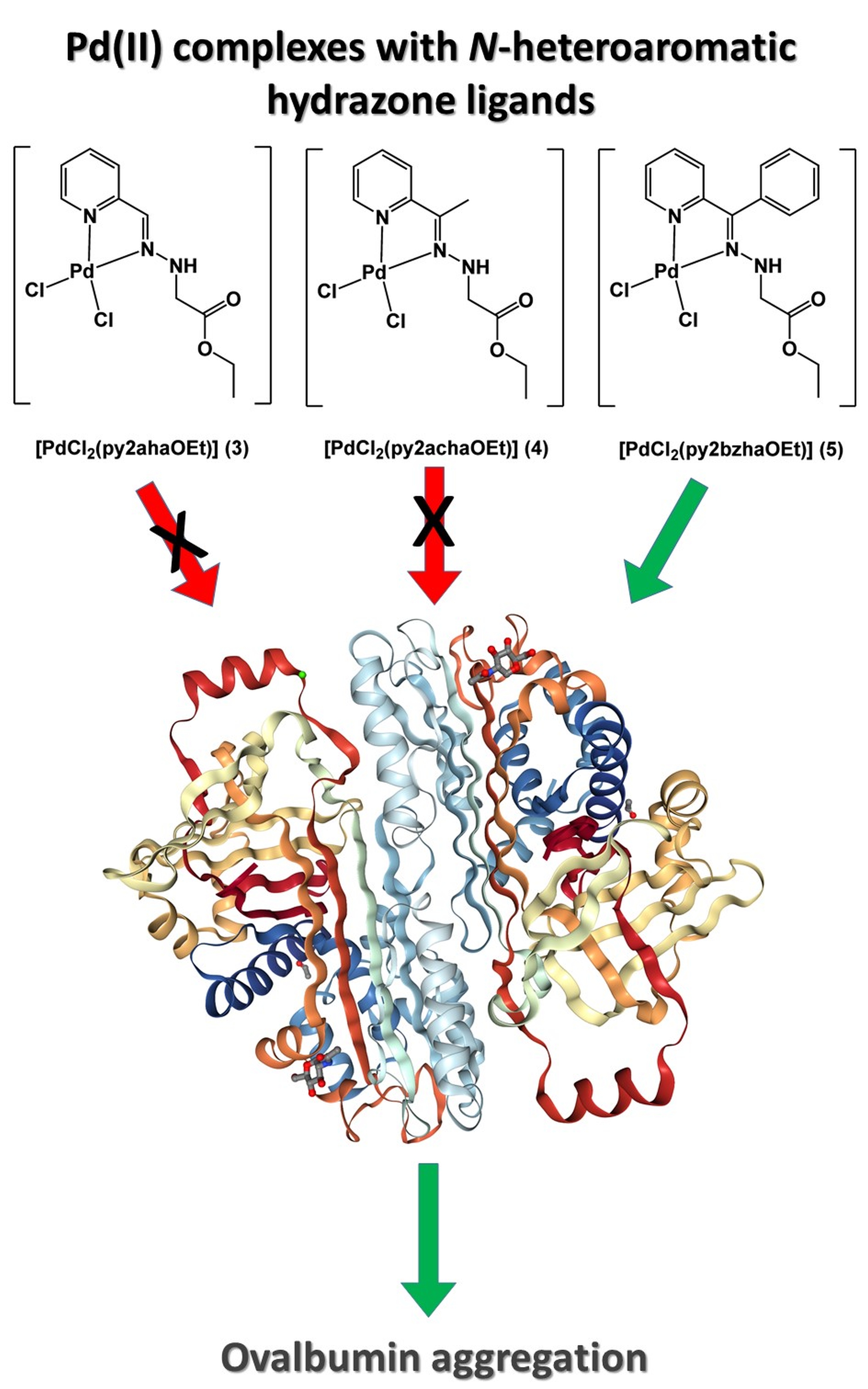
Previously, the cytotoxic actions of five Pd(II) complexes with bidentate N-heteroaromatic chelators (complexes 1–5) on a palette of several cancer cell lines were investigated. However, the results of the cytotoxic activity did not correlate with the hydrophobic character of the complexes. To gain further insight into the structure–activity relationship, essential for the design of novel potential drugs, other factors, such as non-specific interactions with cellular proteins, have to be taken into account. To explore the potential non-specific influence of the complexes on protein structures, ovalbumin (OVA) was chosen as a model system to mimic cellular non-specific crowding environments with high protein concentrations. A Fourier-transform infrared spectroscopy study implied that the binding of 3 and 4 led to only moderate alternations in the secondary structures of the protein, without the possibility to penetrate into hydrophobic core of the protein and disruption of protein native fold. Contrary, the effect of complex 5 on OVA secondary structures was concentration-dependent. While the lower concentration of complex 5 had no effect on OVA structure, a doubled concentration of complex 5 led to complete disruption of the content native-like secondary structures. The concentration-dependent effect of complex 5 on the changes in secondary structures and considerable increase in the exposure of OVA hydrophobic surfaces to water may be related to a potential crosslinking that leads to OVA aggregation.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-68/2022-14/200168;451-03-68/2022-14/200288
References
D. M. Cheff, M. D. Hall, J. Med. Chem. 60 (2017) 4517 (https://doi.org/10.1021/acs.jmedchem.6b01351)
S. Dasari, P. Bernard Tchounwou, Eur. J. Pharmacol. 740 (2014) 364 (https://doi.org/10.1016/j.ejphar.2014.07.025)
N. J. Wheate, S. Walker, G. E. Craig, R. Oun, Dalton Trans. 39 (2010) 8113 (https://doi.org/10.1039/c0dt00292e)
T. C. Johnstone, K. Suntharalingam, S. J. Lippard, Chem. Rev. 116 (2016) 3436 (https://doi.org/10.1021/acs.chemrev.5b00597)
S. Medici, M. Peana, V. M. Nurchi, J. I. Lachowicz, G. Crisponi, M. A. Zoroddu, Coord. Chem. Rev. 284 (2015) 329 (https://doi.org/10.1016/j.ccr.2014.08.002)
M. N. Alam, F. Huq, Coord. Chem. Rev. 316 (2016) 36 (https://doi.org/10.1016/J.CCR.2016.02.001)
A. R. Kapdi, I. J. S. Fairlamb, Chem. Soc. Rev. 43 (2014) 4751 (https://doi.org/10.1039/C4CS00063C)
M. Fanelli, M. Formica, V. Fusi, L. Giorgi, M. Micheloni, P. Paoli, Coord. Chem. Rev. 310 (2016) 41 (https://doi.org/10.1016/j.ccr.2015.11.004)
N. Filipovicć, S. Grubišić, M. Jovanović, M. Dulović, I. Marković, O. Klisurić, A. Marinković, D. Mitić, K. Anđelković, T. Todorović, Chem. Biol. Drug Des. 84 (2014) 333 (https://doi.org/10.1111/cbdd.12322)
S. K. Bjelogrlić, T. R. Todorović, M. Kojić, M. Senćanski, M. Nikolić, A. Višnjevac, J. Araškov, M. Miljković, C. D. Muller, N. R. Filipović, J. Inorg. Biochem. 199 (2019) 110758 (https://doi.org/10.1016/j.jinorgbio.2019.110758)
,A. Daina, O. Michielin, V. Zoete, Sci. Rep. 7 (2017) 42717 (https://doi.org/10.1038/srep42717)
A. Daina, O. Michielin, V. Zoete, J. Chem. Inf. Model. 54 (2014) 3284 (https://doi.org/10.1021/CI500467K)
M. G. Mendoza-Ferri, C. G. Hartinger, M. A. Mendoza, M. Groessl, A. E. Egger, R. E. Eichinger, J. B. Mangrum, N. P. Farrell, M. Maruszak, P. J. Bednarski, F. Klein, M. A. Jakupec, A. A. Nazarov, K. Severin, B. K. Keppler, J. Med. Chem. 52 (2009) 916 (https://doi.org/10.1021/JM8013234)
J. A. Huntington, P. E. Stein, J. Chromatogr., B 756 (2001) 189 (https://doi.org/10.1016/S0378-4347(01)00108-6)
H. Y. Hu, H. N. Du, J. Protein Chem. 19 (2000) 177 (https://doi.org/10.1023/A:1007099502179)
M. Sogami, S. Era, T. Koseki, N. Nagai, J. Pept. Res. 50 (1997) 465 (https://doi.org/10.1111/J.1399-3011.1997.TB01210.X)
C. Lara, S. Gourdin-Bertin, J. Adamcik, S. Bolisetty, R. Mezzenga, Biomacromolecules 13 (2012) 4213 (https://doi.org/10.1021/BM301481V)
J. Li, S. Zhang, C. C. Wang, J. Biol. Chem. 276 (2001) 34396 (https://doi.org/10.1074/JBC.M103392200)
B. Van Den Berg, R. J. Ellis, C. M. Dobson, EMBO J. 18 (1999) 6927 (https://doi.org/10.1093/EMBOJ/18.24.6927)
C. F. MacRae, I. Sovago, S. J. Cottrell, P. T. A. Galek, P. McCabe, E. Pidcock, M. Platings, G. P. Shields, J. S. Stevens, M. Towler, P. A. Wood, J. Appl. Crystallogr. 53 (2020) 226 (https://doi.org/10.1107/S1600576719014092)
N. M. O’Boyle, M. Banck, C. A. James, C. Morley, T. Vandermeersch, G. R. Hutchison, J. Cheminform. 3 (2011) 1 (https://doi.org/10.1186/1758-2946-3-33/TABLES/2)
J. Milošević, J. Petrić, B. Jovčić, B. Janković, N. Polović, Spectrochim. Acta, A 229 (2020) 117882 (https://doi.org/10.1016/J.SAA.2019.117882)
J. Milošević, R. Prodanović, N. Polović, Molecules 26 (2021) 970 (https://doi.org/10.3390/MOLECULES26040970)
C. A. Lipinski, F. Lombardo, B. W. Dominy, P. J. Feeney, Adv. Drug Deliv. Rev. 46 (2001) 3 (https://doi.org/10.1016/S0169-409X(00)00129-0)
C. Aldrich, C. Bertozzi, G. I. Georg, L. Kiessling, C. Lindsley, D. Liotta, K. M. Merz, A. Schepartz, S. Wang, ACS Cent. Sci. 3 (2017) 143 (https://doi.org/10.1021/ACSCENTSCI.7B00069)
T. Sterling J. J. Irwin, J. Chem. Inf. Model. 55 (2015) 2324 (https://doi.org/10.1021/ACS.JCIM.5B00559)
R. Wang, Y. Fu, L. Lai, J. Chem. Inf. Comput. Sci. 37 (1997) 615 (https://doi.org/10.1021/CI960169P)
S. A. Wildman, G. M. Crippen, J. Chem. Inf. Comput. Sci. 39 (1999) 868 (https://doi.org/10.1021/CI990307L)
I. Moriguchi, H. Hirano, I. Nakagome, Chem. Pharm. Bull. 42 (1994) 976 (https://doi.org/10.1248/CPB.42.976)
I. Moriguchi, S. Hirono, Q. Liu, Izum. Nakagome, Y. Matsushita, Chem. Pharm. Bull. 40 (1992) 127 (https://doi.org/10.1248/CPB.40.127)
B. Rašković, N. Babić, J. Korać, N. Polović, J. Serb. Chem. Soc. 80 (2015) 613 (https://doi.org/10.2298/JSC140901007R)
A. Dong, J. D. Meyer, J. L. Brown, M. C. Manning, J. F. Carpenter, Arch. Biochem. Biophys. 383 (2000) 148 (https://doi.org/10.1006/ABBI.2000.2054)
G. Vedantham, H. G. Sparks, S. U. Sane, S. Tzannis, T. M. Przybycien, Anal. Biochem. 285 (2000) 33 (https://doi.org/10.1006/ABIO.2000.4744)
D. Smith, V.B. Galazka, N. Wellner, I. G. Sumner, Int J. Food Sci Tech 35 (2000) 361 (https://doi.org/10.1046/j.1365-2621.2000.00395.x)
J. S. Cristóvão, B. J. Henriques, C. M. Gomes, Methods Mol. Biol. 1873 (2019) 3 (https://doi.org/10.1007/978-1-4939-8820-4_1)
A. Barth, Biochim. Biophys. Acta - Bioenergy 1767 (2007) 1073 (https://doi.org/10.1016/J.BBABIO.2007.06.004)
T. M. Greve, K. B. Andersen, O. F. Nielsen, Spectroscopy 22 (2008) 405 (https://doi.org/10.3233/SPE-2008-0358)
A. N. L. Batista, J. M. Batista, V. S. Bolzani, M. Furlan, E. W. Blanch, Phys. Chem. Chem. Phys. 15 (2013) 20147 (https://doi.org/10.1039/C3CP53525H)
P. Huang, A. Dong, W. S. Caughey, J. Pharm. Sci. 84 (1995) 387 (https://doi.org/10.1002/JPS.2600840402)
S. Roy, B. Jana, B. Bagchi, J. Chem. Phys. 136 (2012) 115103 (https://doi.org/10.1063/1.3694268)
A. Tjernberg, N. Markova, W. J. Griffiths, D. Hallén, J. Biomol. Screen. 11 (2006) 131 (https://doi.org/10.1177/1087057105284218)
T. Arakawa, Y. Kita, S. N. Timasheff, Biophys. Chem. 131 (2007) 62 (https://doi.org/10.1016/J.BPC.2007.09.004)
S. Tunçer, R. Gurbanov, I. Sheraj, E. Solel, O. Esenturk, S. Banerjee, Sci. Rep. 8 (2018) 14828 (https://doi.org/10.1038/s41598-018-33234-z)
C. X. Wang, F. F. Yan, Y. X. Zhang, L. Ye, J. Photochem. Photobiol., A 192 (2007) 23 (https://doi.org/10.1016/J.JPHOTOCHEM.2007.04.032)
E. Schönbrunn, S. Eschenburg, K. Luger, W. Kabsch, N. Amrhein, Proc. Natl. Acad. Sci. U.S.A. 97 (2000) 6345 (https://doi.org/10.1073/PNAS.120120397)
M. S. Celej, G. G. Montich, G. D. Fidelio, Protein Sci. 12 (2003) 1496 (https://doi.org/10.1110/PS.0240003).